Figure 4. DabA contains a β-CA-like active site but is not active outside of the membrane.
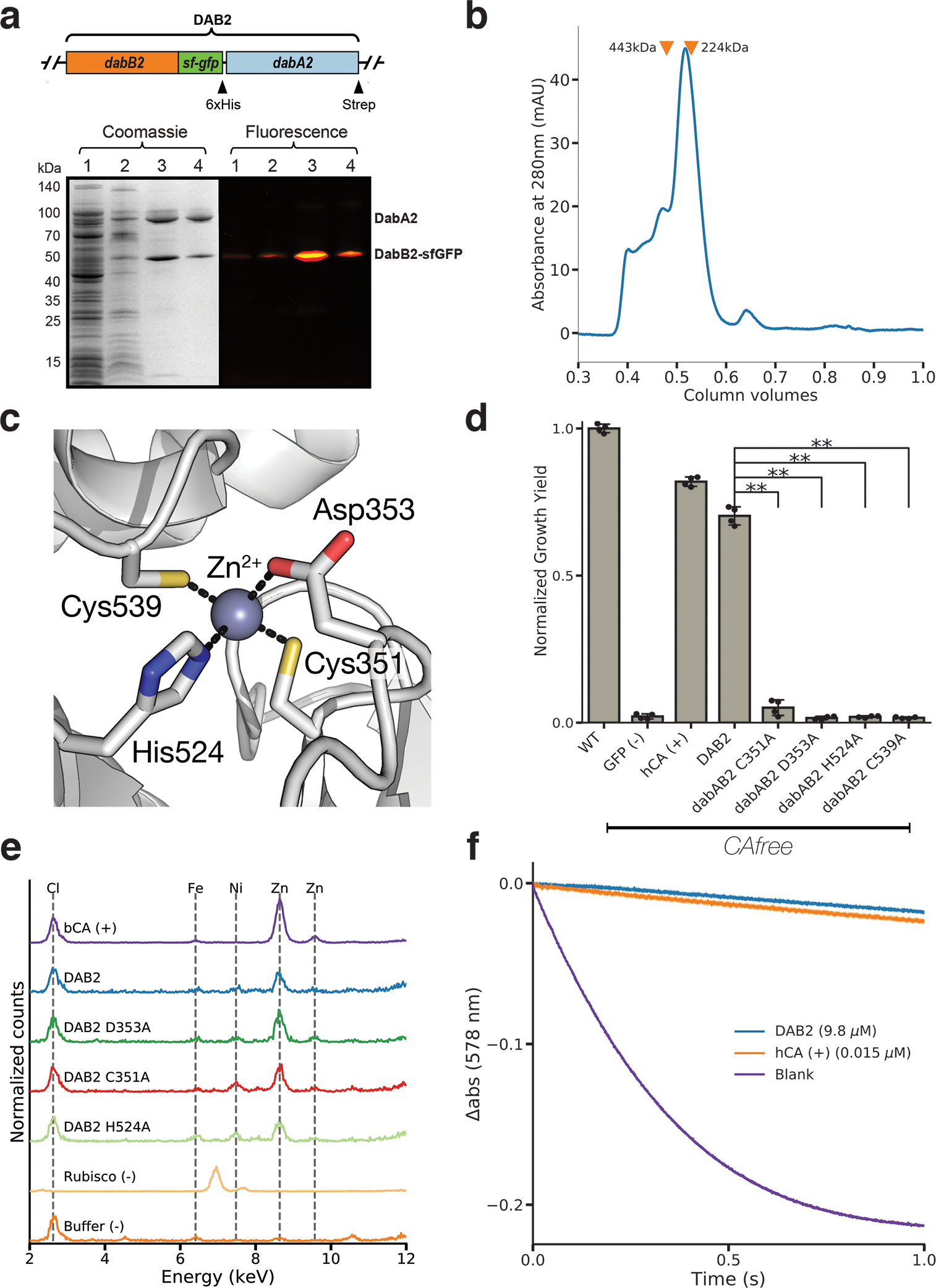
a. Purification of DabAB2 complex from E. coli. DabA2 was C-terminally tagged with a Strep-tag and DabB2 was C-terminally tagged with sf-GFP and a 6xHis-tag. Purification was monitored using SDS-PAGE imaged with fluorescence (right view) before coomassie staining (left view). Lane 1: clarified lysate; 2: solubilized membranes; 3: Ni-NTA resin eluent; 4: strep-tactin resin eluent. DabA2 and DabB2 co-purify as a single complex without any obvious interactors. Similar results were observed after 3 independent purifications. b. Size-exclusion chromatogram of His/Strep purified DabAB2 with retention volumes (orange arrows) and molecular weights (kDa) indicated for standard samples (apoferritin, 443 kDa; β-amylase, 224 kDa). DabAB2 runs with an effective mass of ~270 kDa, which likely reflects an oligomer of DabA and DabB. Similar results were observed after 3 independent purifications. c. Structural model of the DabA2 active site based on a β-CA from E. coli (PDB 1I6P). Typical β-CAs rely on two cysteines and one histidine to bind Zn2+. The aspartic acid coordinates Zn2+ but is likely displaced during catalysis43. d. Alanine mutants of the putative DabA2 active site residues abrogate rescue of CAfree E. coli compared to wild-type dabAB2 (C351A, t=54.3, p=1.1*10−8; D353A, t=144, p=3.1*10−11; H524A, t=44, p=3.7*10−8; C539A, t=44.3, p=3.5*10−8; all p values listed here are Bonferroni corrected). “**” denotes that means are significantly different with Bonferroni corrected p < 5X10−4 according to a two-tailed t-test. Bar heights indicate means and error bars give standard deviations of four biological replicate cultures. e. X-ray fluorescence data indicate that DabAB2 binds zinc like all known β-CAs. Single mutations to the active site do not abrogate zinc binding. Curves are from representative samples. Technical replicate traces were concordant (WT: 9, D353A:5, H524A:4, C351A:3, Rubisco: 2, Blank: 4, BCA: 3). Replicate traces for DAB2 H524A include samples from two independent purifications. f. Purified DabAB2 does not display any obvious CA activity despite being present in 650-fold excess over the positive control (Human carbonic anhydrase II, hCA) in our assays. Curves display averages of 7 experimental traces +/− standard error of the mean. Similar results were observed in two independent purifications.
