Figure 6. A speculative model of the unidirectional energy-coupled CA activity of DAB complexes.
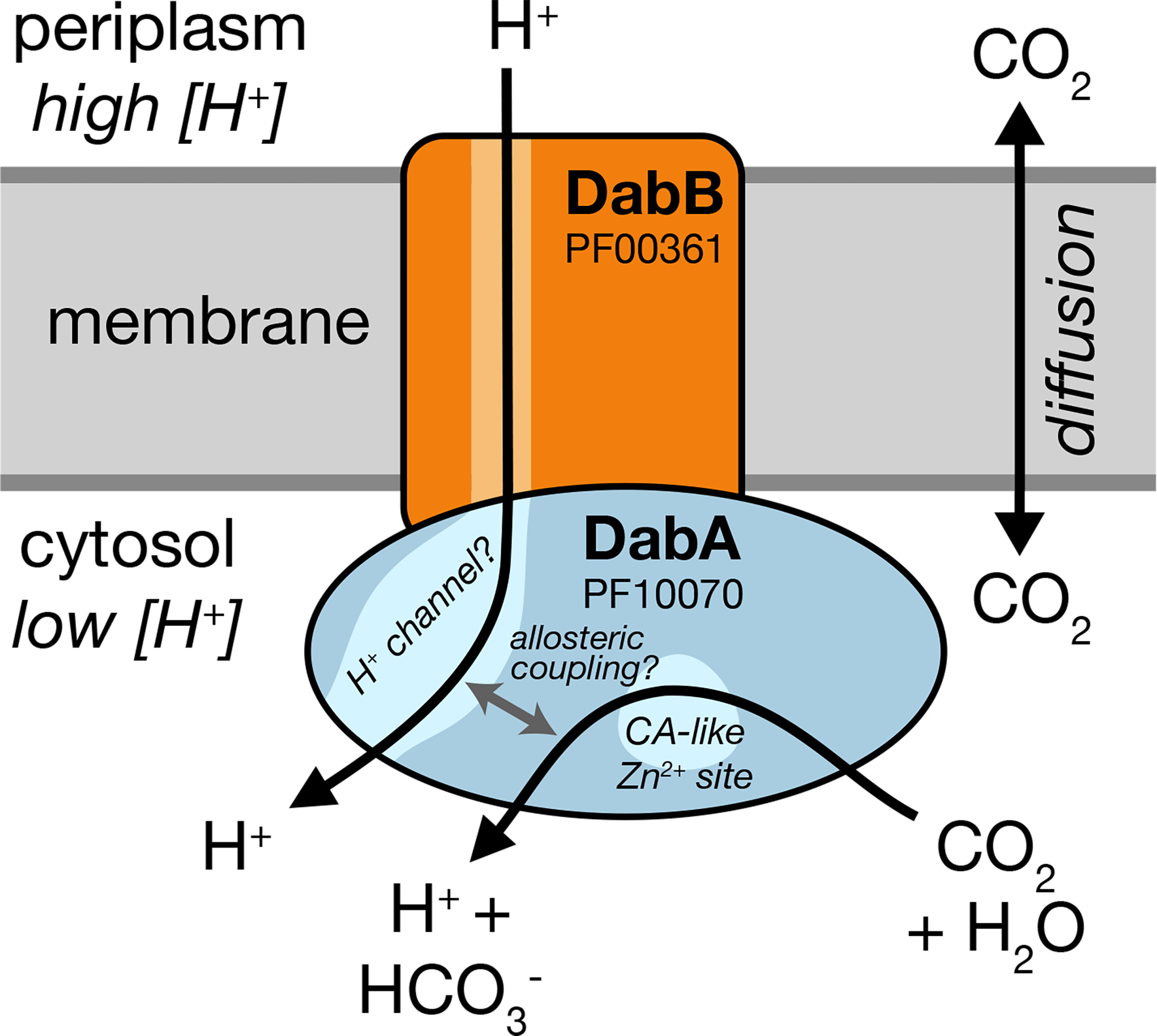
We propose that DabAB complexes couple CA activity of DabA to a cation gradient across the cell membrane, producing unidirectional hydration of CO2 to HCO3−. The cation gradient could be H+ or Na+. Energy-coupled CA activity is required for the DABs role as a Ci uptake system in the proteobacterial CCM, as discussed in the text. Because it appears that DabAB2 is not active as a purified complex outside of the membrane, it is assumed protein tightly couples the inflow of cations with CO2 hydration so that there is no “slippage.” Indeed, slippage - i.e., uncoupled CA activity - would be counterproductive for CCM function7,14. Notably, Zn2+ binding by the active site aspartic acid of type II β-CAs (D353 in DabA2, Figure 4a) is thought to allosterically regulate activity43. This Asp-mediated activity switch could, therefore, provide a means for allosteric coupling of a β-CA active site to distal ion transport.
