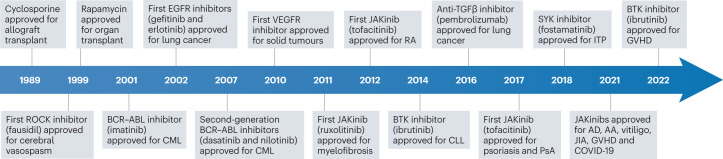
Fig. 2. Timeline of approval of key protein kinase inhibitor drugs for cancer and immune-mediated disease.
The timeline lists the year of approval and indication, starting with the approval of cyclosporine for allograft transplantation (in 1989) to the approvals of JAK inhibitors (JAKinibs) for immune-mediated diseases to date. AA, alopecia areata; AD, atopic dermatitis; BCR, B cell receptor; BTK, Bruton’s tyrosine kinase; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; EGFR, epidermal growth factor receptor; GVHD, graft versus host disease; ITP, immune thrombocytopenia; JAK, Janus kinase; JIA, juvenile idiopathic arthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; ROCK; RHO-associated kinase; SYK, spleen tyrosine kinase; TGFβ, transforming growth factor-β; VEGFR, vascular endothelial growth factor receptor.