Abstract
Lumbar canal stenosis (LCS) has been reported as a precipitating factor by which a tethered spinal cord, which is asymptomatic during childhood, develops into tethered cord syndrome (TCS) in adulthood. However, only a few reports on surgical strategies for such cases are available. A 64-year-old woman presented with unbearable pain in the left buttock and dorsal aspect of the thigh approximately 1 year ago. Magnetic resonance imaging showed cord tethering with a filar-type spinal lipoma and LCS due to the thickening of the ligamentum flavum at the L4-5 vertebral level. Five months after the decompressive laminectomy for the treatment of LCS, an untethering surgery was performed at the dural cul-de-sac at the S4 level. The severed end of the filum was elevated rostrally by 7 mm, and the pain subsided postoperatively. This case study shows that surgeries for both lesions should be indicated for adult-onset TCS triggered by LCS.
Keywords: filar lipoma, lumbar canal stenosis, tethered spinal cord syndrome, untethering
Introduction
Tethered spinal cord syndrome (TCS) is a developmental abnormality that impairs the longitudinal movement of the spinal cord and that can be combined with various forms of spinal dysraphism. Although TCS usually occurs in childhood, a tethered spinal cord can be asymptomatic for decades until adulthood, when it may become symptomatic.1-9) When Pang et al.7) first described adult-onset TCS, they cited three precipitating factors as the mechanism by which TCS develops in adulthood. These factors include 1) transit stretching of the spine, 2) mechanical constriction/narrowing of the spinal canal, and 3) spinal trauma. The mechanisms causing spinal canal narrowing include lumbar canal stenosis (LCS) due to lumbar spondylosis/spondylolisthesis and intervertebral disc protrusion.2,3,6-9) When LCS is involved in adult-onset TCS, both an untethering surgery and a decompressive surgery for LCS may be necessary, but only a few studies have reported the appropriate surgical strategy because reports of such cases are rare.6,7) Herein, we report a case of a presenile patient with a filar lipoma in whom a successful untethering surgery was performed 5 months after decompressive laminectomy for LCS.
Case Report
A 64-year-old woman presented to our hospital approximately a year ago with unbearable pain in the left buttock and dorsal aspect of the thigh. She also complained of lumbago, which had simultaneously developed. Both pains did not worsen with postural change, but she complained of worsening after long walking. Neurologically, she had very mild hypesthesia in the lateral part of the left dorsalis pedis and equivocal weakness in the left anterior tibial, extensor hallucis, and digitorum longus muscles. A neurogenic bladder was ruled out because her pre- and postvoiding echography did not reveal an enlarged bladder or residual urine. Magnetic resonance imaging (MRI) showed cord tethering with a spinal lipoma (Fig. 1a, b). The dural cul-de-sac was located at the S4 vertebral level, where the filar lipoma was terminated. Severe LCS due to the thickening of the ligamentum flavum at the L4-5 vertebral level was also noted (Fig. 1a, c).
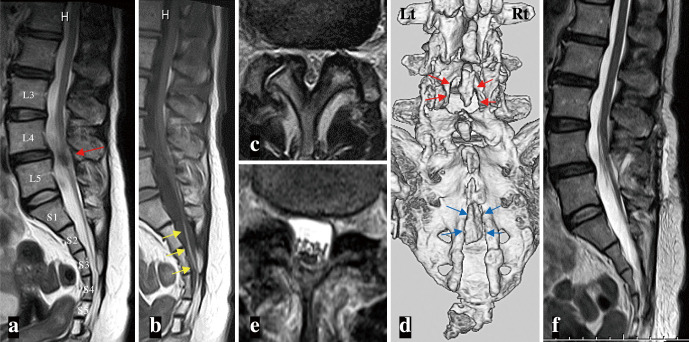
Fig. 1.
(a-c) Midsagittal views of T2-weighted (T2WI) (a) and T1-weighted magnetic resonance images (b), and axial view of T2WI (c) at the L4-5 vertebral level (a, red arrow) demonstrate spinal cord tethering with a filar-type spinal lipoma (b, yellow arrows) and lumbar canal stenosis (LCS) due to the thickening of the ligamentum flavum.
(d) Postoperative three-dimensional (3D) reconstruction of a bone-targeted computed tomographic scan shows the extent of the decompressive laminectomy at L4-5 with the preservation of the spinous process (red arrows). An ectopic bone covering the spina bifida at the S3 and upper part of the S4 level is also noted (blue arrows).
(e-f) A month after decompressive surgery for LCS, an axial view of T2WI (e) reveals a successfully enlarged dural sac at the L4-5 level. No change is noted in the degree of spinal cord tethering on a midsagittal view of T2WI (f).
Because these pains did not improve with medical treatment for 6 months, a surgery was indicated. First, for the LCS at the L4-5 level, the patient underwent spinous process-splitting laminectomy of the lower two-thirds part of L4 and the upper one-third part of L5 (Fig. 1d). Postoperatively, her lumbago and minute positive neurological findings subsided, but the pain in her left buttock and dorsal thigh persisted. Postoperative MRI confirmed an enlarged dural sac at the L4-5 level with successful decompressive laminectomy (Fig. 1e), but no change was noted in the degree of cord tethering (Fig. 1f).
Five months after the initial surgery, she underwent a second surgery. Following the resection of the ectopic bone covering the spina bifida at the S3 and S4 levels (Fig. 1d), a fatty filum with a diameter of 5 mm, which terminated slightly to the left of the midline of the dural cul-de-sac at the S4 level, was exposed (Fig. 2a). The stimulation of the filum did not evoke a muscle response in the lower limbs and anus. In addition, we confirmed that nerve roots did not arise from the filum. To untether the cord, the filum was severed at the rostral part of the operative field, and the severed end of the filum was elevated rostrally by 7 mm (Fig. 2b). The caudal part of the filum was also severed at the dural cul-de-sac and resected as a 5-mm long column.
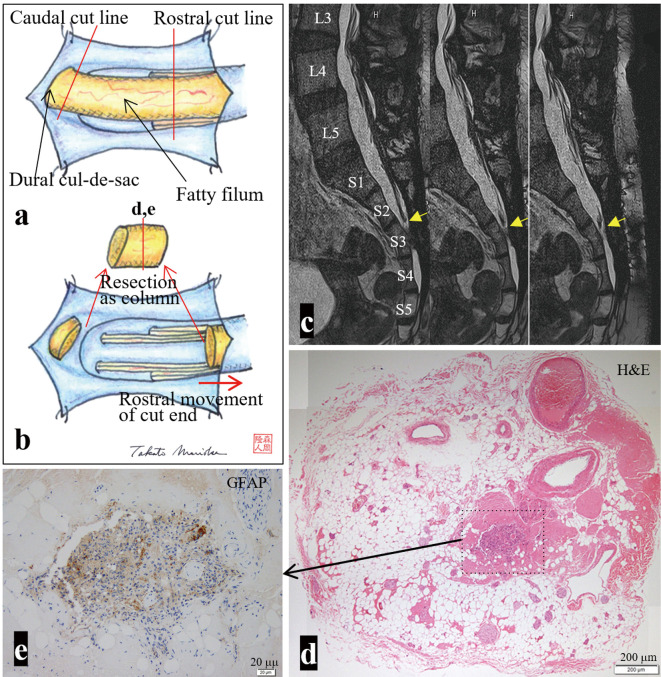
Fig. 2.
(a, b) Schematic drawing of the operative findings. (a) Dural opening at the S3 and S4 vertebral levels reveals a fatty filum terminated slightly to the left of the midline of the dural cul-de-sac. (b) The filum is severed at the rostral part of the operative field, and the severed end of the filum is elevated rostrally. The caudal part of the filum is also severed at the dural cul-de-sac and resected as a column. (c) Four months after the second surgery, serial sagittal views of 3D heavily T2WI (slice thickness, 1 mm) show a successful untethering surgery. The severed end is located at the upper part of S3 vertebral level (yellow arrows). (d, e) On the axial section of the resected filum, histopathologically, glial fibrillary acidic protein (GFAP)-positive neuroglial tissue is observed in the center of the fibroadipose tissue. The location of the section is indicated as a red line in (b). The area of the enlarged view is indicated as a dotted square in (d). Hematoxylin and eosin (H&E).
Postoperatively, the pain in her left buttock and dorsal thigh subsided over a month. Postoperative MRI confirmed a successful untethering surgery (Fig. 2c). The histopathological examination of the resected filum showed glial fibrillary acidic protein (GFAP)-positive neuroglial tissue in the center of the fibroadipose tissue. However, no central canal-like structures were observed (Fig. 2d, e).
Discussion
Considering the clinical course of the present case, cord tethering by a filar lipoma, which had been asymptomatic until the 60s, developed into TCS and presented as unbearable left buttock and dorsal thigh pain. The TCS was triggered by the mechanical constriction/narrowing of the spinal canal with LCS, as initially advocated by Pang et al.7) The painful buttock and dorsal thigh on the left side corresponded to the left S2-3 region on the dermatome. Therefore, this pain could not be explained by the presence of LCS at the L4-5 level as a result of the first surgery. The fact that the symptoms improved for the first time after the untethering surgery also supports this theory.
In terms of the appropriate surgical strategy in such cases, recent authors10-12) have demonstrated improvement in symptoms only after decompressive surgery for the treatment of LCS. However, the symptoms in these cases were due to compression myelopathy with LCS instead of TCS. In cases with TCS, similar to the present case, a surgery for both lesions is considered necessary.6) When both surgical sites coincided, it is possible to perform both surgeries in one stage.6) However, in this case, we believed that the least-invasive untethering surgery could be performed at the S3-4 level near the dural cul-de-sac, and we planned a two-staged surgery. However, no studies have reported which operation should be first performed. LCS forms later than congenital cord tethering, and the mechanical constriction of the spinal canal due to LCS is severe, suggesting that decompressive surgery alone may improve symptoms. Therefore, in this case, priority was given to decompressive surgery for LCS, which can be performed by only epidural manipulation. However, TCS symptoms did not improve, and an untethering surgery was performed after 5 months. As the severed end of the filum was elevated rostrally by 7 mm after the untethering and the symptoms improved, we believe that performing the decompressive surgery first was useful.
The reason that TCS manifested by LCS does not improve with only successful decompression is not clear and remains within the realm of speculation. It is generally believed that the pathophysiology of TCS is related most of all to chronic ischemia of the cord.3,4,8,9,13) We speculate that, also in this case, chronic ischemia, which has existed subclinically for over 60 years, was aggravated by LCS and manifested the symptoms. This chronic ischemia persisted even after the successful decompression for the LCS, presumably necessitating the untethering surgery.
The retained medullary cord (RMC) was first proposed by Pang et al.,14) in which a cord-like structure (C-LS) is continuous from the conus medullaris and extends to the dural cul-de-sac, resulting in cord tethering. In this decade, several additional reports regarding RMC have been published.15-19) RMC is believed to originate from the late arrest of secondary neurulation.14) The characteristic histopathological findings of C-LS include the presence of a central canal-like ependyma-lined canal with surrounding GFAP-positive neuroglial tissues in a fibrocollagenous tissue, corroborating a remnant of the cavitary medullary cord, which is typically destined to regress.14) Recently, some studies reported that this histopathological finding was present frequently in filar and caudal lipomas, which are also believed to originate from the secondary neurulation failure.18,19) Because central canal-like structures are not present from end to end of RMC tissue,15-18) central canal-like structures were missing in this case whereas GFAP-positive neuroglial tissues were present. The present findings provided further evidence for the idea that RMC and filar lipomas can be considered consequences of the continuum of regression failure during secondary neurulation.14,15,18,19)
Conclusion
The present case suggests that surgery for both lesions should be indicated for adult-onset TCS triggered by LCS.
Conflicts of Interest Disclosure
The authors have no conflict of interest to declare.
Acknowledgments
We would like to thank Dr. Nobuya Murakami and Dr. Ai Kurogi, Department of Neurosurgery, Fukuoka Children's Hospital, and Dr. Nobutaka Mukae and Dr. Takafumi Shimogawa, Department of Neurosurgery, Graduate School of Medicine, Kyushu University, for critically reading this manuscript. We would like to thank Editage for editing a draft of this manuscript.
References
- 1). Düz B, Gocmen S, Secer HI, Basal S, Gönül E: Tethered cord syndrome in adulthood. J Spinal Cord Med 31: 272-278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Hüttmann S, Krause J, Collmann H, Sörensen N, Roosen K: Surgical management of tethered spinal cord in adults: report of 54 cases. J Neurosurg 95: 173-178, 2001 [DOI] [PubMed] [Google Scholar]
- 3). Iskandar BJ, Fulmer BB, Hadley MN, Oakes WJ: Congenital tethered spinal cord syndrome in adults. Neurosurg Focus 10: e7, 2001 [DOI] [PubMed] [Google Scholar]
- 4). Klekamp J: Tethered cord syndrome in adults. J Neurosurg Spine 15: 258-270, 2011 [DOI] [PubMed] [Google Scholar]
- 5). Lee GY, Paradiso G, Tator CH, Gentili F, Massicotte EM, Fehlings MG: Surgical management of tethered cord syndrome in adults: indication, techniques, and long-term outcomes in 60 patients. J Neurosurg Spine 4: 123-131, 2006 [DOI] [PubMed] [Google Scholar]
- 6). Martinez-Lage JF, Piqueras C, Poza M: Lumbar canal stenosis. A cause of late neurological deterioration in patients with spina bifida. Surg Neurol 55: 256-260, 2001 [DOI] [PubMed] [Google Scholar]
- 7). Pang D, Wilberger JE Jr: Tethered cord syndrome in adults. J Neurosurg 57: 32-47, 1982 [DOI] [PubMed] [Google Scholar]
- 8). Rajpal S, Tubbs RS, George T, et al. : Tethered cord due to spina bifida occulta presenting in adulthood: a tricenter review of 61 patients. J Neurosurg Spine 6: 210-215, 2007 [DOI] [PubMed] [Google Scholar]
- 9). Stetler WR Jr, Park P, Sullivan S: Pathophysiology of adult tethered cord syndrome: review of the literature. Neurosurg Focus 29: E2, 2010 [DOI] [PubMed] [Google Scholar]
- 10). Breton JM, Yang MJ, Riesenburger RI: The use of decompressive segmental sublaminoplasty to treat myelopathy caused by lumbar stenosis in tethered cord syndrome. J Surg Case Rep 3: 1-3, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Hashimoto K, Tsubakino T, Hoshikawa T, et al. : Elderly-onset degenerative lumbar spondylotic myelopathy in a patient with a low-placed spinal cord successfully treated by laminotomy: a case report. Clin Case Rep 3: 1021-1025, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Srinivas S, Shetty R, Collins I: Symptomatic lumbar disc protrusion causing progressive myelopathy in a low-lying cord. Global Spine J 2: 115-118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Yamada S, Zinke DE, Sanders D: Pathophysiology of “tethered cord syndrome”. J Neurosurg 54: 494-503, 1981 [DOI] [PubMed] [Google Scholar]
- 14). Pang D, Zovickian J, Moes GS: Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery 68: 1500-1519, 2011 [DOI] [PubMed] [Google Scholar]
- 15). Morioka T, Murakami N, Suzuki SO, Nakamura R, Mizoguchi M: Subpial lipoma associated with retained medullary cord. NMC Case Rep J 8: 51-55, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Morioka T, Murakami N, Kanata A, Tsukamoto E, Suzuki OS: Retained medullary cord associated with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst 36: 423-427, 2020 [DOI] [PubMed] [Google Scholar]
- 17). Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO: Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg 55: 380-387, 2020 [DOI] [PubMed] [Google Scholar]
- 18). Morioka T, Murakami N, Suzuki SO, et al. : Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. Surg Neurol Int 12: 1-8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Morioka T, Murakami N, Kurogi A, et al. : Embryopathological relationship between retained medullary cord and caudal spinal lipoma. Interdicip Neurosurg 29: 101534, 2022 [Google Scholar]