Figure 1.
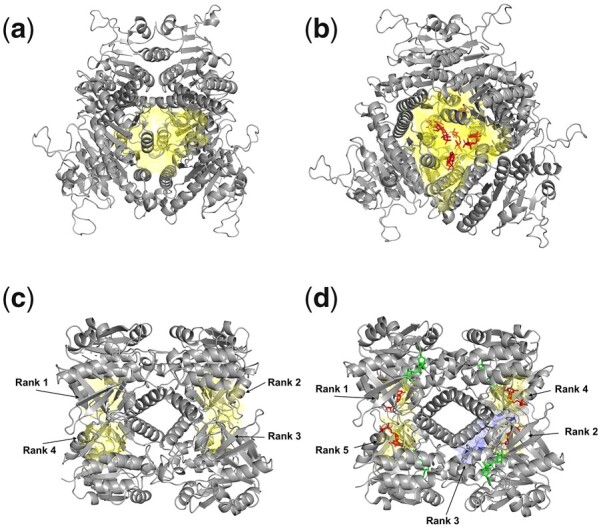
Validation by APOP for known allosteric pockets in uridylate kinase and glucose-1-phosphate thymidylyltransferase structures, for both apo and holo conformations. (a) apo form of uridylate kinase (PDB ID: 3EK6) where a known allosteric pocket is predicted as the rank 1 pocket by APOP from among a total of 88 pockets in the structure, (b) holo form of uridylate kinase (PDB ID: 3EK5) where the known allosteric pocket is predicted as rank 1 pocket by APOP from among the 84 pockets in the structure. (c) apo state of glucose-1-phosphate thymidylyltransferase (PDB ID: 1FZW) where the known allosteric pocket is predicted as the rank 1 pocket by APOP from among the total of 66 pockets in the structure, (d) holo state of glucose-1-phosphate thymidylyltransferase (PDB ID: 1H5T) where the known allosteric pocket is predicted as the rank 1 pocket by APOP from the total of 60 pockets in the structure. Allosteric ligands are shown in red, and the corresponding allosteric pockets predicted by APOP are shown in yellow. Substrates are colored green.
