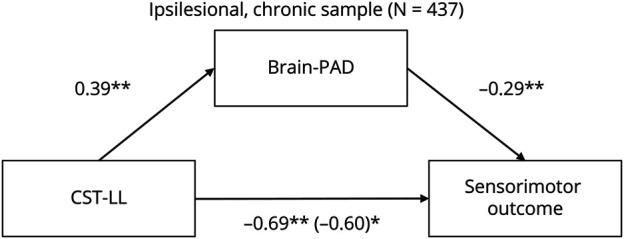
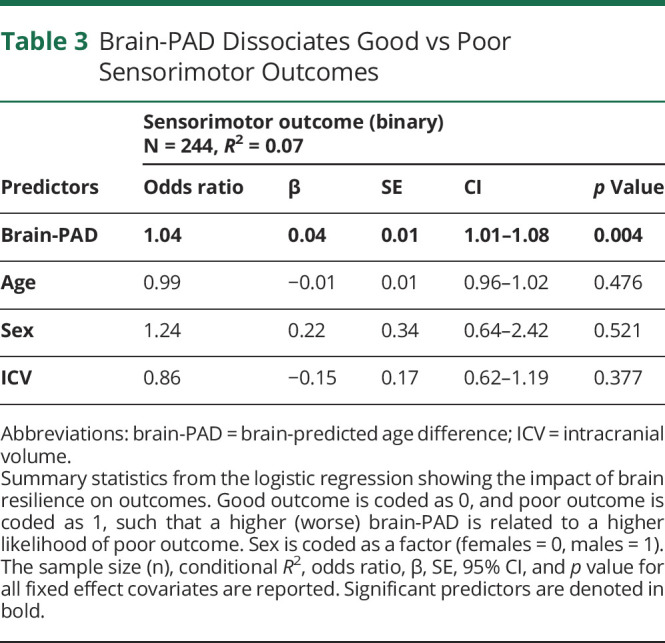
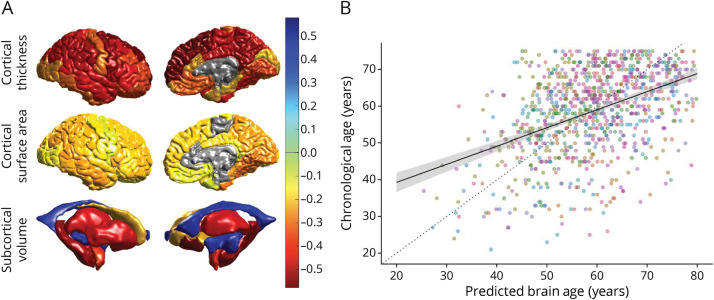
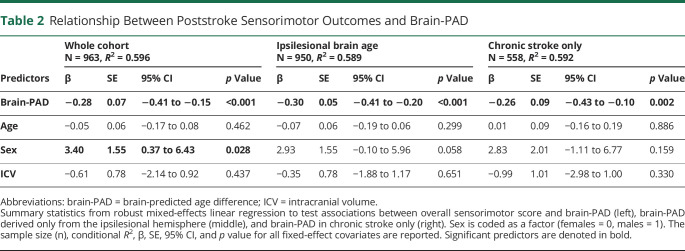
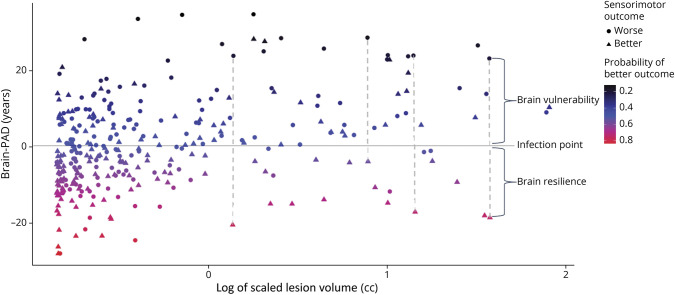
Sook-Lei Liew
Sook-Lei Liew, PhD OTR/L
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,✉,
Nicolas Schweighofer
Nicolas Schweighofer, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
James H Cole
James H Cole, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Artemis Zavaliangos-Petropulu
Artemis Zavaliangos-Petropulu, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Bethany P Lo
Bethany P Lo, BSc
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Laura KM Han
Laura KM Han, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,✉,
Tim Hahn
Tim Hahn, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Lianne Schmaal
Lianne Schmaal, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Miranda R Donnelly
Miranda R Donnelly, MS
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Jessica N Jeong
Jessica N Jeong, BA
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Zhizhuo Wang
Zhizhuo Wang, MSc
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Aisha Abdullah
Aisha Abdullah, BA
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Jun H Kim
Jun H Kim, BSc
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Alexandre Hutton
Alexandre Hutton, MEng
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Giuseppe Barisano
Giuseppe Barisano, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Michael R Borich
Michael R Borich, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Lara A Boyd
Lara A Boyd, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Amy Brodtmann
Amy Brodtmann, MBBS, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Cathrin M Buetefisch
Cathrin M Buetefisch, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Winston D Byblow
Winston D Byblow, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Jessica M Cassidy
Jessica M Cassidy, DPT, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Charalambos C Charalambous
Charalambos C Charalambous, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Valentina Ciullo
Valentina Ciullo, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Adriana Bastos Conforto
Adriana Bastos Conforto, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Rosalia Dacosta-Aguayo
Rosalia Dacosta-Aguayo, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Julie A DiCarlo
Julie A DiCarlo, MS
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Martin Domin
Martin Domin, Dr rer med
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Adrienne N Dula
Adrienne N Dula, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Natalia Egorova-Brumley
Natalia Egorova-Brumley, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Wuwei Feng
Wuwei Feng, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Fatemeh Geranmayeh
Fatemeh Geranmayeh, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Chris M Gregory
Chris M Gregory, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Colleen A Hanlon
Colleen A Hanlon, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Kathryn Hayward
Kathryn Hayward, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Jess A Holguin
Jess A Holguin, OTD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Brenton Hordacre
Brenton Hordacre, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Neda Jahanshad
Neda Jahanshad, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Steven A Kautz
Steven A Kautz, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Mohamed Salah Khlif
Mohamed Salah Khlif, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Hosung Kim
Hosung Kim, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Amy Kuceyeski
Amy Kuceyeski, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
David J Lin
David J Lin, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Jingchun Liu
Jingchun Liu, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Martin Lotze
Martin Lotze, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Bradley J MacIntosh
Bradley J MacIntosh, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
John L Margetis
John L Margetis, OTD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Maria Mataro
Maria Mataro, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Feroze B Mohamed
Feroze B Mohamed, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Emily R Olafson
Emily R Olafson, BSc
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Gilsoon Park
Gilsoon Park, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Fabrizio Piras
Fabrizio Piras, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Kate P Revill
Kate P Revill, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Pamela Roberts
Pamela Roberts, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Andrew D Robertson
Andrew D Robertson, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Nerses Sanossian
Nerses Sanossian, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Heidi M Schambra
Heidi M Schambra, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Na Jin Seo
Na Jin Seo, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Surjo R Soekadar
Surjo R Soekadar, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Gianfranco Spalletta
Gianfranco Spalletta, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Cathy M Stinear
Cathy M Stinear, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Myriam Taga
Myriam Taga, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Wai Kwong Tang
Wai Kwong Tang, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Greg T Thielman
Greg T Thielman, PT, MSPT, ATC, EdD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Daniela Vecchio
Daniela Vecchio, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Nick S Ward
Nick S Ward, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Lars T Westlye
Lars T Westlye, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Carolee J Winstein
Carolee J Winstein, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
George F Wittenberg
George F Wittenberg, MD, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Steven L Wolf
Steven L Wolf, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Kristin A Wong
Kristin A Wong, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Chunshui Yu
Chunshui Yu, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Steven C Cramer
Steven C Cramer, MD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1,
Paul M Thompson
Paul M Thompson, PhD
1From the Laboratory of Neuro Imaging (G.B.), Imaging Genetics Center (N.J., P.M.T.), and Department of Neurology (N. Sanossian, C.J.W.), Mark and Mary Stevens Neuroimaging and Informatics Institute (S.-L.L., J.H.K., H.K., G.P.), Keck School of Medicine, University of Southern California, Los Angeles; Chan Division of Occupational Science and Occupational Therapy (S.-L.L., B.P.L., M.R.D., J.N.J., Z.W., A.A., A.H., J.A.H., J.L.M.), Division of Biokinesiology and Physical Therapy (N. Schweighofer, C.J.W., S.-L.L.), and Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; Centre for Medical Image Computing (J.H.C.), Department of Computer Science, Dementia Research Centre (J.H.C.), Queen Square Institute of Neurology, University College London, United Kingdom; Brain Mapping Center (A.Z.-P.), Department of Neurology, Geffen School of Medicine, University of California, Los Angeles; Centre for Youth Mental Health (L.K.M.H., L.S.) and Melbourne School of Psychological Sciences (N.E.-B.), University of Melbourne, Parkville, Victoria, Australia; Institute for Translational Psychiatry (T.H.), University of Münster, Germany; Department of Physical Therapy and Djavad Mowafaghian Centre for Brain Health (L.A.B.), University of British Columbia, Vancouver, Canada; The Florey Institute of Neuroscience and Mental Health (A.B., K.H., M.S.K.); Eastern Cognitive Disorders Clinic (A.B.); Royal Melbourne Hospital (A.B.), Victoria, Australia; Department of Neurology (C.M.B.) and Facility for Education and Research in Neuroscience (K.P.R.), Emory University, Atlanta, GA; Centre for Brain Research and Department of Exercise Sciences (W.D.B.), University of Auckland, New Zealand; Department of Health Sciences (J.M.C.), University of North Carolina at Chapel Hill; Department of Basic and Clinical Sciences (C.C.C.), Center for Neuroscience and Integrative Brain Research (CENIBRE) (C.C.C.), University of Nicosia Medical School, Cyprus; Laboratory of Neuropsychiatry (V.C., F.P.), IRCCS Santa Lucia Foundation, Rome, Italy; Hospital das Clínicas (A.B.C.), São Paulo University; Hospital Israelita Albert Einstein (A.B.C.), São Paulo, Brazil; Department of Psychiatry and Clinical Psychobiology (R.D.-A.), Universitat de Barcelona, Spain; Center for Neurotechnology and Neurorecovery (J.A.D., D.J.L.), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston; Functional Imaging Unit (M.D.), Diagnostic and Neuroradiology and Functional Imaging Unit (M.L.), Diagnostic Radiology and Neuroradiology, Universitymedicine Greifswald, Germany; Departments of Neurology (A.N.D.) and Physical Medicine & Rehabilitation (K.A.W.), Dell Medical School at The University of Texas Austin; Department of Neurology (W.F.), Duke University School of Medicine, Durham, NC; Clinical Language and Cognition (CLC) Lab (F.G.), Department of Brain Sciences, Imperial College London, United Kingdom; Department of Health Sciences & Research (C.M.G., S.A.K.), Medical University of South Carolina, Charleston; Cancer Biology (C.A.H.), Wake Forest School of Medicine, Winston Salem, NC; Innovation, Implementation and Clinical Translation (IIMPACT) in Health (B.H.), Allied Health and Human Performance, University of South Australia, Adelaide; Ralph H. Johnson VA Medical Center (S.A.K., N.J.S.), Charleston, SC; Department of Radiology (A.K., E.R.O.), Weill Cornell Medicine, New York, NY; Department of Radiology (J.L., C.Y.), Tianjin Medical University General Hospital, China; Hurvitz Brain Sciences Program (B.J.M.), Centre for Brain Resilience & Recovery and Canadian Partnership for Stroke Recovery (A.D.R.), Sunnybrook Research Institute, Toronto, Ontario, Canada; Computational Radiology Artificial Intelligence Unit (B.J.M.) and Norwegian Centre for Mental Disorders Research (NORMENT) (L.T.W.), Department of Mental Health and Addiction, Oslo University Hospital, Norway; Department of Clinical Psychology and Psychobiology (M.M.), and Institut de Neurociències (M.M.), University of Barcelona; Institut de Recerca Pediàtrica (M.M.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain; Jefferson Integrated MRI Center (F.B.M.), Department of Radiology, Thomas Jefferson University, Philadelphia, PA; Cedars-Sinai (P.R.), Los Angeles, CA; California Rehabilitation Institute (P.R., S.C.C.), Los Angeles; Department of Kinesiology and Health Sciences (A.D.R.), University of Waterloo, Ontario, Canada; Department of Neurology (H.M.S., M.T.), NYU Langone, New York; Department of Rehabilitation Sciences (N.J.S.), and Department of Health Sciences and Research (N.J.S.), Medical University of South Carolina, Charleston; Department of Psychiatry and Neurosciences (S.R.S.), Clinical Neurotechnology Laboratory, Charité–Universitätsmedizin Berlin, Germany; Laboratory of Neuropsychiatry (G.S.), IRCCS Santa Lucia Foundation, Rome, Italy; Division of Neuropsychiatry (G.S.), Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX; Department of Medicine (C.M.S.), University of Auckland, New Zealand; Department of Psychiatry (W.K.T.), Faculty of Medicine, The Chinese University of Hong Kong; Department of Physical Therapy and Neuroscience (G.T.T.), University of the Sciences, Philadelphia, PA; Department of Clinical and Behavioral Neurology (D.V.), IRCCS Santa Lucia Foundation, Rome, Italy; UCL Queen Square Institute of Neurology (N.S.W.), London, United Kingdom; Department of Psychology (L.T.W.), University of Oslo, Norway; Department of Neurology & RNEL (G.F.W.), University of Pittsburgh; GRECC/HERL (G.F.W.), VA Pittsburgh Healthcare System, PA; Department of Rehabilitation Medicine Division of Physical Therapy (M.R.B., S.L.W.), Department of Rehabilitation Medicine, Division of Geriatric Medicine (S.L.W.), Department of Medicine, and Department of Cell Biology (S.L.W.), Emory University School of Medicine; Center for Visual and Neurocognitive Rehabilitation (S.L.W.), Atlanta VA Health Care System, GA; and Department of Neurology (S.C.C.), University of California, Los Angeles. Giuseppe Barisano is currently at the department of Neurosurgery, Stanford Medicine, Stanford University, CA; Kathryn Hayward is currently at the departments of Physiotherapy and Medicine (RMH), University of Melbourne, Parkville, Victoria, Australia; and Gregory T. Thielman is currently at Physical Therapy and Neuroscience, School of Health Professions, Saint Joseph's University, Philadelphia, PA.
1