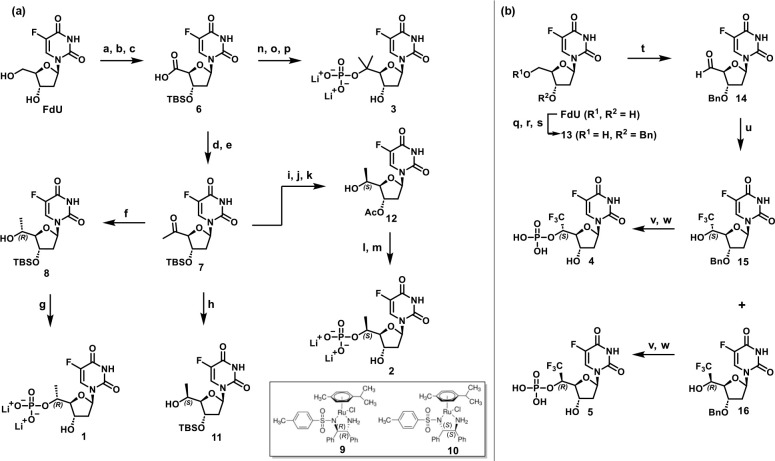
Scheme 1. Synthesis of 5′-Functionalized FdUMP Analogs.
Reagents and conditions: (a) TBSCl, imidazole, DMAP, DMF, rt, 87%; (b) PPTS, MeOH, rt, 67%; (c) TEMPO, PhI(OAc)2, MeCN/THF/H2O, rt, 85%; (d) Me(OMe)NH-HCl, T3P, EtOAc/pyridine, rt (e) MeMgBr, THF, −78°C, 71%, over 2 steps; (f) 9, HCO2Na, H2O/EtOAc, rt, 75%, 98:2 dr; (g) i, POCl3, pyridine, MeCN/H2O, 0°C to rt; ii, NH4F, H2O, rt; iii, Dowex-Li+, rt, 7%; (h) 10, HCO2Na, H2O/EtOAc, rt, 83%, 7:3 dr; (i) TBAF, THF, 0°C; (j) Ac2O, DMAP, pyridine, rt, 47% over 2 steps; (k) 10, HCO2Na, H2O/EtOAc, rt, 67%, 99:1 dr; (l) POCl3, pyridine, MeCN/H2O, 0°C to rt, 20%; (m) i, NH3(aq), H2O, rt; ii, Dowex-Li+, rt, 27% over 2 steps; (n) TMSCHN2, toluene, MeOH, rt, 86%; (o) MeMgBr, THF, 0°C to rt, 72%; (p) i, POCl3, trimethyl phosphate, 0°C to rt, 14%; ii, Dowex-Li+, rt, 12%; (q) TrCl, pyridine, μwave, 100°C, 78%; (r) NaH, BnBr, THF, rt, 67%; (s) 80% AcOH/H2O, rt, 81%; (t) DMP, DCM, rt; (u) TMSCF3, TBAF, THF, 0 °C to rt, then 0.5 N HCl, rt, silica gel chromatography (EtOAc/hexanes; 0–80%), 45% over 2 steps, 15 (S), 27%; 16 (R), 18%; (v) POCl3, pyridine, MeCN/H2O, 0°C to rt; (w) Pd(OH)2, H2, MeOH, rt (4 (S), 12%, over 2 steps; 5 (R), 6%, over 2 steps).