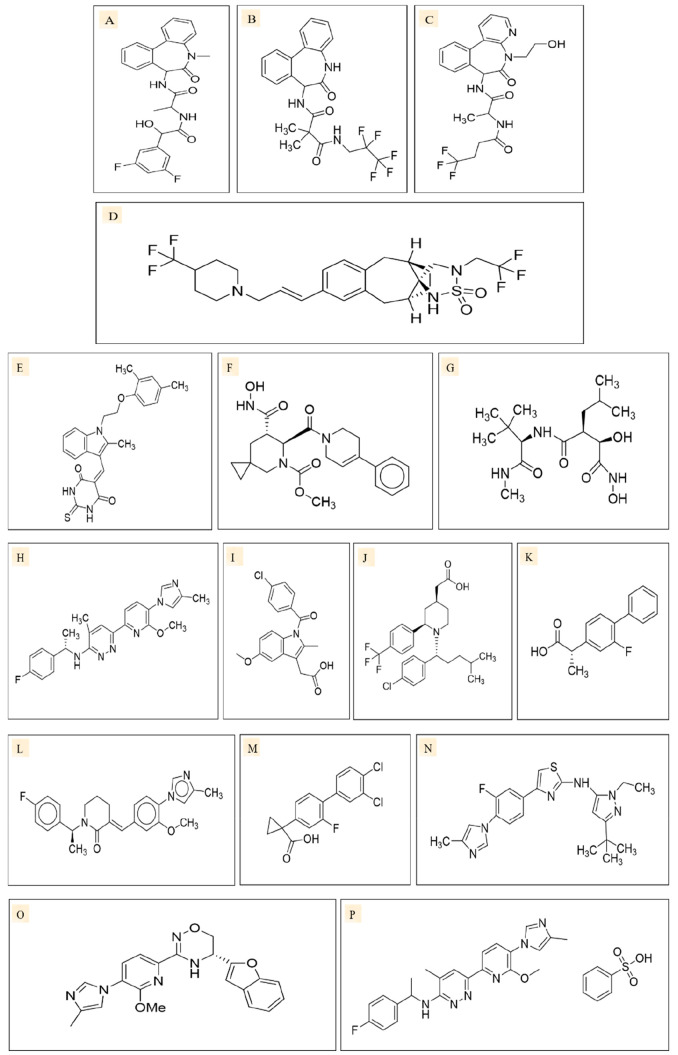
Figure 8.
Two-dimensional structures of γ-secretase inhibitors of different classes: (i) azepine (transition state analogues), e.g., LY411575 (A), and azepine (nontransition state analogues), e.g., DAPT (B); (ii) sulfonamides (nontransition state analogues), e.g., MK0752 (C); and (iii) peptide isoesterase (transition state analogues), e.g., GSI-1 (D). Two-dimensional structures of ADAM inhibitors, such as ZLDI-8 (E), INCB3619 (F), and Marimastat (G). Two-dimensional structures of γ-secretase modulators such as BPN15606 (H), Indomethacin (I), GSM-1 (J), Tarenflurbil (K), E2012 (L), CHF5074 (M), SGSM36 (N), FRM36143 (O), and BPN15606 (P).