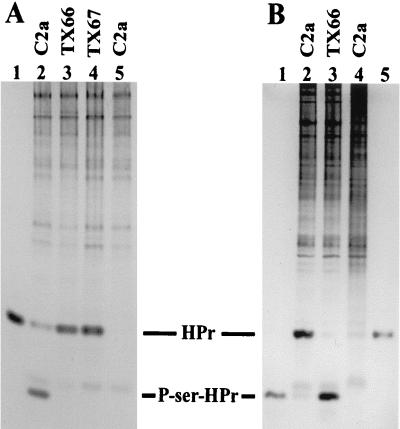
FIG. 3.
HPr kinase and P-Ser-HPr phosphatase activity in cell extracts of S. xylosus wild-type and hprK mutant strains. (A) HPr kinase activity in cell extracts of S. xylosus wild-type C2a and the hprK mutant strains TX66 and TX67. His-15-Ala HPr from S. aureus served as the substrate. HPr forms were separated together with whole cell extracts on 15% nondenaturing polyacrylamide gels and visualized by Coomassie blue staining. The assays were carried out with 15 μg of cell extracts, 3 μg of HPr, and 5 mM ATP in 50 mM Tris (pH 7.5)–50 mM NaCl–10 mM MgCl2 for 20 min at 37°C. Lanes 1 (3 μg of HPr) and 5 (15 μg of wild-type [C2a] extract, no HPr), controls; lane 2, S. xylosus wild-type (C2a) extract; lane 3, TX66 (hprK::ermB) extract; lane 4, TX67 (hprK::ermB) extract. The positions of HPr and P-Ser-HPr are indicated. (B) P-Ser-HPr phosphatase activity in cell extracts of S. xylosus wild-type C2a and the hprK mutant strain TX66. The P-Ser derivative of S. aureus His-15-Ala-HPr served as the substrate. HPr forms were separated together with whole cell extracts on 15% nondenaturing polyacrylamide gels and visualized by Coomassie blue staining. The assays were carried out with 40 μg of cell extracts, 4 μg of P-Ser-HPr, and 1 mM NaH2PO4, in 50 mM Tris (pH 7.5)–50 mM NaCl–10 mM MgCl2 for 20 min at 37°C. Lane 1 (1 μg of HPr), lane 4 (S. xylosus wild-type extract, no P-Ser-HPr), and lane 5 (1 μg of P-Ser-HPr), controls; lane 2, S. xylosus wild-type (C2a) extract; lane 3, TX66 (hprK::ermB) extract. The positions of HPr and P-Ser-HPr are indicated.