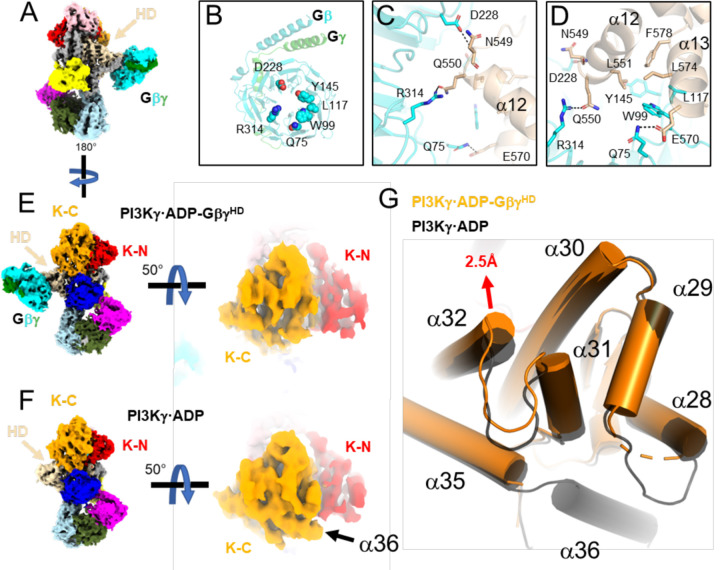
Figure 2. Release of the tryptophan lock and α36 upon Gβγ binding to the p110γ HD.
(A) Cryo-EM map of PI3Kγ·ADP–GβγHD with domains colored as in Fig. 1A. (B) Gβγ residues involved in the p110γ helical domain (HD) interaction. (C-D) Residues in the Gβγ–HD interface, shown as sticks and colored according to their respective domains. (E-F) Comparison of the maps for PI3Kγ·ADP–GβγHD and PI3Kγ·ADP. Density for α36 is not observed in PI3Kγ·ADP–GβγHD. (G) Overlay of K-C in PI3Kγ·ADP–GβγHD (orange) and PI3Kγ·ADP (black). The movement of the α32 N-terminus is 2.5 Å at the Cα of Leu1004.