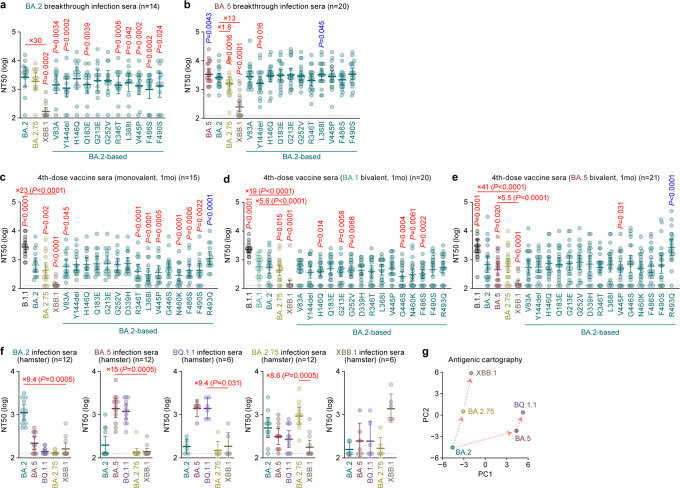
Fig. 2. Immune resistance of XBB.1.
Neutralization assays were performed with pseudoviruses harboring the spike (S) proteins of B.1.1, BA.1, BA.2, BA.5, BQ.1.1, BA.2.75 and XBB.1. The BA.2 S-based derivatives are included in (a–e). The following sera were used. Convalescent sera from fully vaccinated individuals who had been infected with BA.2 after full vaccination (9 2-dose vaccinated and 5 3-dose vaccinated. 14 donors in total) (a), and BA.5 after full vaccination (2 2-dose vaccinated donors, 17 3-dose vaccinated donors and 1 4-dose vaccinated donors. 20 donors in total) (b). 4-dose vaccine sera collected at 1 month after the 4-dose monovalent vaccine (15 donors) (c), BA.1 bivalent vaccine (20 donors) (d), and BA.5 bivalent vaccine (21 donors) (e). f Sera from hamsters infected with BA.2 (12 hamsters), BA.5 (12 hamsters), BQ.1.1 (6 hamsters), BA.2.75 (12 hamsters), and XBB.1 (6 hamsters). g Antigenic cartography based on the results of neutralization assays using hamster sera (Fig. 2f). Assays for each serum sample were performed in triplicate to determine the 50% neutralization titer (NT50). Each dot represents one NT50 value, and the geometric mean and 95% confidential interval (CI) are shown. Statistically significant differences were determined by two-sided Wilcoxon signed-rank tests. The P values versus BA.2 (a), BA.5 (b), or XBB.1 (c–f) are indicated in the panels. For the BA.2 derivatives (a–e), statistically significant differences (P < 0.05) versus BA.2 are indicated with asterisks. Red and blue asterisks, respectively, indicate decreased and increased NT50s. The horizontal dashed line indicates the detection limit (120-fold). Information on the convalescent donors is summarized in Supplementary Table 5. Source data are provided with this paper.