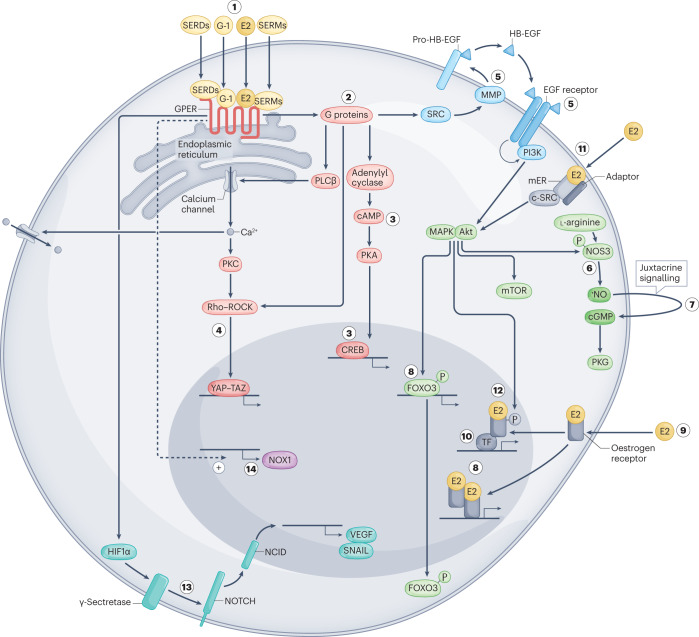
Fig. 1. Cellular signalling pathways activated by ERα, ERβ and GPER.
Non-genomic and genomic signalling pathways are activated by oestrogen and oestrogenic ligands (in yellow) through binding to the three known oestrogen receptors, oestrogen receptor-α (ERα), oestrogen receptor-β (ERβ) and the G protein-coupled oestrogen receptor (GPER). 17β-Oestradiol (E2), selective agonists such as G-1, or selective oestrogen receptor modulators (SERMs) and selective oestrogen receptor downregulators and/or degraders (SERDs) activate GPER (1), which is localized predominantly intracellularly at the endoplasmic reticulum. GPER activates several heterotrimeric G proteins (2), leading to multiple downstream cascades, including cAMP production (3) and activation of PKA (3) and CREB (3). G protein activation also leads to calcium (Ca2+) mobilization from intracellular stores, which activates PKC and leads to activation of plasma membrane calcium channels. GPER activation can also lead to regulation of gene expression via activation of the YAP–TAZ transcription factors via Rho–ROCK signalling (4). Activation of SRC via G proteins can also lead to activation of matrix metalloproteinases (MMPs) (5) that cleave pro-heparin-binding epidermal growth factor (HB-EGF) (5), releasing free HB-EGF. HB-EGF then transactivates the EGF receptor (5), which in turn activates MAPK (ERK1/2), Akt and other pathways. These induce additional, rapid (non-genomic) effects such as activation of the l-arginine–endothelial nitric oxide synthase (NOS3)–NO–cGMP pathway (in combination with mobilization of calcium stores). Akt causes phosphorylation of endothelial NOS3 (6), which releases nitric oxide (NO) and leads to juxtacrine signalling from endothelial to vascular smooth muscle cells (7), and activation of PKG. Activation of MAPK and Akt signalling also causes genomic effects regulating gene transcription such as FOXO3 phosphorylation and degradation (8). In the classic, genomic oestrogen receptor pathway, 17β-oestradiol binds cytosolic and nuclear oestrogen receptors (9), inducing receptor dimerization and binding to the promoters of target genes. Alternatively, activated oestrogen receptors modulate the function of other classes of transcription factors (TF) through protein–protein interactions (10). Subpopulations of membrane-bound oestrogen receptors (mER) are present at the plasma membrane (11). Once activated, these oestrogen receptors interact with adaptor proteins (adaptor) and signalling molecules, such as SRC, which mediate rapid signalling events (for example, PI3K–Akt and MAPK signalling) (11). Oestrogen receptor ERα, potentially following transactivation of EGFR by GPER, is regulated by phosphorylation through kinases (such as MAPK and Akt), resulting in the regulation of gene expression (12). HIF1α, following GPER activation, induces γ-secretase-dependent activation of NOTCH (13) and VEGF signalling (13). Basal expression and/or activity of GPER constitutively induces expression of the NADPH oxidase NOX1 (14).