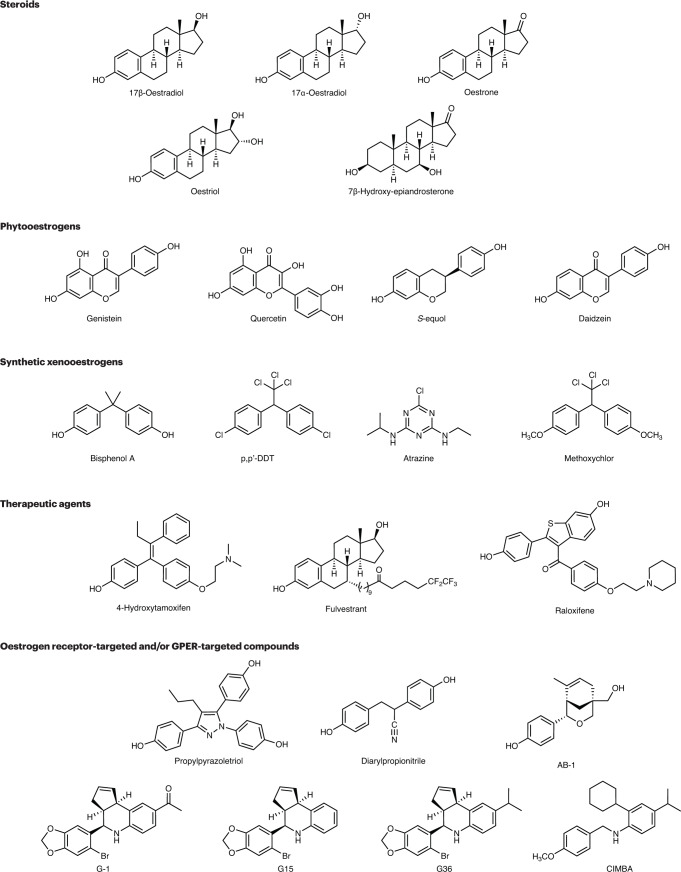
Fig. 2. Chemical structures of compounds that act as ligands for ERα, ERβ and/or GPER.
Shown are examples of natural steroids, phytooestrogens, xenooestrogens/endocrine disrupting chemicals (EDCs), therapeutic agents and experimental compounds that display varying activities towards oestrogen receptor-α (ERα), oestrogen receptor-β (ERβ) and the G protein-coupled oestrogen receptor (GPER) but are generally non-selective. Also shown are synthetic experimental compounds that exhibit selectivity for ERα and/or ERβ, such as propylpyrazoletriol (PPT), diarylpropionitrile (DPN) and AB-1, or for GPER, such as G-1, G15, G36 and CIMBA. p,p′-DDT, p,p′-dichlorodiphenyltrichloroethane.