Abstract
Introduction:
Tumour-emitted molecules induce immunosuppression in the tumour microenvironment. An immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO/IDO1), facilitates immune escape in several malignant tumours, including osteosarcoma. Upregulation of IDO establishes a tolerogenic environment in the tumour and the tumour-draining lymph nodes. IDO-induced downregulation of effector T-cells and upregulation of local regulatory T-cells creates immunosuppression and promotes metastasis.
Observations:
Osteosarcoma is the most common bone tumour characterised by immature bone formation by the tumour cells. Almost 20% of osteosarcoma patients present with pulmonary metastasis at the time of diagnosis. The improvement in therapeutic modalities for osteosarcoma has been in a stagnant phase for two decades. Therefore, the development of novel immunotherapeutic targets for osteosarcoma is emergent. High IDO expression is associated with metastasis and poor prognosis in osteosarcoma patients.
Conclusion and Relevance:
At present, only a few studies are available describing IDO’s role in osteosarcoma. This review describes the prospects of IDO not only as a prognostic marker but also as an immunotherapeutic target for osteosarcoma.
Keywords: Indoleamine 2,3-dioxygenase; immunosuppression; immunotherapeutic target; osteosarcoma
Introduction
Osteosarcoma is the most common primary malignancy of the bone.[1] It has a high tendency for local invasion and metastasis.[1] Osteosarcoma is highly heterogeneous by nature.[2-5] The genomic processes driving the oncogenesis of osteosarcoma are still unrevealed.[2] The current therapeutic options for osteosarcoma patients are a combined chemotherapy regimen and surgery, but the prognosis of metastatic or recurrent osteosarcoma is still disappointing.[1,6] Developing a novel immune checkpoint target may provide hope for metastatic osteosarcoma patients. The immune system plays a pivotal role in osteosarcoma disease progression.[7] Novel immunotherapeutic approaches have been investigated to improve survival.[7] Understanding the involvement of the immune system in osteosarcoma might help in improving patient outcomes.[7] Hence, osteosarcoma prognosis may be related to immune system functional status.
Tumour cells can escape the immune attack through several mechanisms.[8-11] One of the immunosuppressive mechanisms is the up-regulation of indoleamine 2,3-dioxygenase (IDO). IDO is an intracellular enzyme that degrades tryptophan into kynurenine.[12] High expression of IDO in the tumour and tumour-draining lymph nodes has been observed in breast cancer, colon cancer, melanoma, ovarian cancer, brain tumours, soft-tissue sarcoma, acute myelogenous leukemia and several other cancers.[13-26] IDO inhibits effector T-cells by depleting the essential amino acid tryptophan and augmenting the production of kynurenine metabolites.[12,27] The IDO enzyme is also involved in the induction of differentiation and maturation of regulatory T-cells.[27] Overexpression of IDO induces tolerance and immunosuppression.[28] In cancer, IDO1 expression has been observed in tumour cells and in the tumour microenvironment, which includes endothelial cells, immune cells, fibroblasts and mesenchymal cells.[29,30] Nevertheless, the pathophysiology of IDO in the tumour microenvironment has been explicated.[30] In this review, we look at the role of IDO in osteosarcoma.
Materials and Methods
Evidence acquisition
The search approach applied the following keywords: IDO and osteosarcoma. Three electronic databases (PubMed/MEDLINE, SCOPUS and GOOGLE SCHOLAR) were searched for articles published between 2002 and 2022. The most relevant and specific articles were extracted from the literature.
IDO in osteosarcoma
It has been established that IDO emerged from cancer and can constrain antitumor immunity. It has been observed that IDO is expressed in solid tumours such as osteosarcoma. The role of IDO in the pathogenesis of osteosarcoma is shown in Table 1.
Table 1.
Review of IDO involvement in osteosarcoma
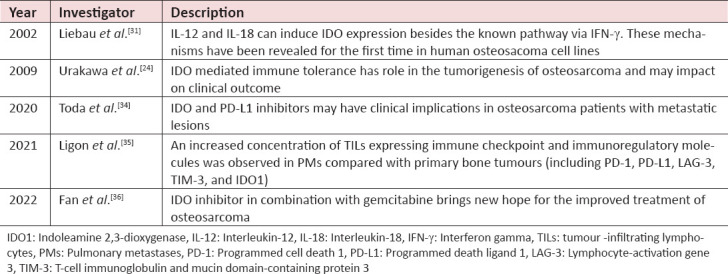
Liebau et al., evaluated the IDO as a new adjuvant therapy for osteosarcoma. Using several cytokines, they investigated the IDO induction in human osteosarcoma cell lines (MNNG/HOS, KHOS-240, HOS and MG-63). Furthermore, they analysed IDO expression in these cell lines in activated lymphocytes in the presence or absence of cytokines. They revealed that the IDO activity in osteosarcoma cell lines (HOS and MG-63) increased in the presence of IL-12 and IL-18, besides the established pathway through IFN-γ. These mechanisms were identified for the first time in human osteosarcoma cell lines.[31]
Urakawa et al., investigated the expression of IDO and its involvement in the prognosis of osteosarcoma. They determined for the first time that osteosarcoma patients with high IDO expression had a worse clinical outcome. They performed the immunohistochemical analysis on the human tissue specimens of 47 patients with high-grade osteosarcoma. The majority of the cases expressed IDO. Their findings showed that IDO has the potential to be a prognostic marker and immunotherapeutic target for osteosarcoma.[24]
A remarkable advancement has been observed in the field of immunotherapy. Meanwhile, an interest in immunotherapy for osteosarcoma has developed recently. Clinical trials have been conducted to check the efficacy of PD-1/PD-L1 (programmed cell death 1/programmed death ligand 1) inhibitors for treating sarcomas, but the therapeutic response in advanced osteosarcoma patients was unsatisfactory.[32] Hence, Harrison and Schwartz suggested that a combinational therapeutic regimen involving immune checkpoint inhibitors and conventional cytotoxic agents is required for these patients.[33] A recent study explored the relationships between IDO1 and PD-L1 expression in 56 osteosarcoma patients. They performed the immunohistochemistry on formalin-fixed, paraffin-embedded tumour tissues to analyse the expression of IDO1 and PD-L1 and compared it with clinicopathological characteristics and prognosis of the osteosarcoma patients. Moreover, they evaluated the effect of IFN-γ on IDO1 and PD-L1 mRNA expression in human osteosarcoma cell lines (U2OS, SaOS2, MG63 and MNNG). It was observed that IDO and PD-L1 expression was significantly higher in those patients who had already received neoadjuvant chemotherapy than those patients who were treatment naïve. The study concluded that IDO and PD-L1 immune checkpoint inhibitors might be clinically beneficial for osteosarcoma patients with metastasis.[34]
Ligon et al., investigated the expression of IDO, PD-1, PD-L1, lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin domain-containing protein 3 in the tissue specimen of 66 osteosarcoma patients. They revealed that the expression of IDO, PD-1, PD-L1, LAG-3 and TIM-3 was significantly higher in the pulmonary metastases of the patients as compared to the primary tumours. They observed that expression of these molecules in the pulmonary metastases was linked with worse progression-free survival. They demonstrated that the microenvironment of metastatic osteosarcoma is highly immunosuppressive due to the upregulation of several checkpoint molecules, tumour-associated macrophages and myeloid-derived suppressor cells. They proposed that their findings would provide combinations of agents to develop next-generation clinical trials for osteosarcoma immunotherapy.[35] In a recent study, a group of researchers demonstrated the effect of IDO inhibitor D-1-Methyltryptophan (D-1-MT) in combinational therapy with Gemcitabine (Gem). This gives new hope for the treatment of osteosarcoma patients.[36]
Conclusion
The tumour microenvironment of osteosarcoma is highly immunosuppressive. IDO is well known immunosuppressive enzyme that is upregulated in several cancers, including osteosarcoma [Figure 1].[13-26,24,31] IDO inhibitors as adjuvant therapeutic agents may have clinical implications in osteosarcoma.[36] Since immunosuppression is a hallmark of cancer and a considerable obstacle to cancer immunotherapy. IDO has the potential to be one of the favorable therapeutic candidates for osteosarcoma. Further studies with larger cohorts are warranted to identify the relationship between IDO upregulation and worse prognosis-free survival in metastatic osteosarcoma patients.
Figure 1.
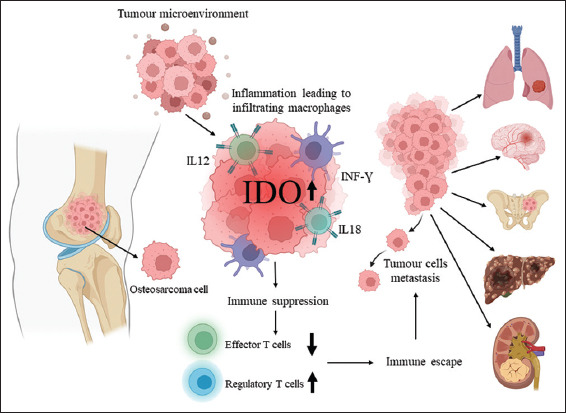
IDO-induced immunosuppression in osteosarcoma. IDO is an immunosuppressive enzyme. IFN-γ is a potent inducer of IDO. IL-12 and IL-18 can also induce the IDO in the tumour microenvironment. IDO inhibits effector T-cell immunity and induces upregulation of regulatory T-cells. High IDO expression is linked with tumour immune escape. IDO-induced immunosuppression might promote metastasis in osteosarcoma patients.
Authors’ Contributions
Conceived and designed the analysis: AF and KA; Collected the data: AF, BZ and KA; Contributed data or analysis tools: BZ; Performed the analysis: AF and KA; Wrote the paper: AF, BZ and KA
Competing interest:
Nil.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma:Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. doi: 10.1016/j.canlet.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma-connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–91. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz S, Barøy T, Sun J, Nome T, Vodák D, Bryne JC, et al. Unscrambling the genomic chaos of osteosarcoma reveals extensive transcript fusion, recurrent rearrangements and frequent novel TP53 aberrations. Oncotarget. 2016;7:5273–88. doi: 10.18632/oncotarget.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A. 2014;111:E5564–73. doi: 10.1073/pnas.1419260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovac M, Blattmann C, Ribi S, Smida J, Mueller NS, Engert F, et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun. 2015;6:8940. doi: 10.1038/ncomms9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment:A complex but targetable ecosystem. Cells. 2020;9:976. doi: 10.3390/cells9040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt HG, Justin EM, Lindsey BA. Applying osteosarcoma immunology to understand disease progression and assess immunotherapeutic response. Adv Exp Med Biol. 2020;1258:91–109. doi: 10.1007/978-3-030-43085-6_6. [DOI] [PubMed] [Google Scholar]
- 8.Messerschmidt JL, Prendergast GC, Messerschmidt GL. How cancers escape immune destruction and mechanisms of action for the new significantly active immune therapies:Helping nonimmunologists decipher recent advances. Oncologist. 2016;21:233–43. doi: 10.1634/theoncologist.2015-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer:Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Menter T, Tzankov A. Mechanisms of immune evasion and immune modulation by lymphoma cells. Front Oncol. 2018;8:54. doi: 10.3389/fonc.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 12.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, et al. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag Res. 2019;11:475–81. doi: 10.2147/CMAR.S184221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlini G, Di Gennaro P, Mariotti G, Urso C, Chiarugi A, Pimpinelli N, et al. Indoleamine 2,3-dioxygenase+cells correspond to the BDCA2+plasmacytoid dendritic cells in human melanoma sentinel nodes. J Invest Dermatol. 2010;130:898–901. doi: 10.1038/jid.2009.307. [DOI] [PubMed] [Google Scholar]
- 16.Speeckaert R, Vermaelen K, Van Geel N, Autier P, Lambert J, Haspeslagh M, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. 2012;48:2004–11. doi: 10.1016/j.ejca.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Ferdinande L, Decaestecker C, Verset L, Mathieu A, Lopez XM, Negulescu AM, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141–7. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer:Effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 19.Zhai L, Lauing KL, Chang AL, Dey M, Qian J, Cheng Y, et al. The role of IDO in brain tumor immunotherapy. J Neurooncol. 2015;123:395–403. doi: 10.1007/s11060-014-1687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–9. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 21.Chamuleau ME, Van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93:1894–8. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 22.Folgiero V, Goffredo BM, Filippini P, Masetti R, Bonanno G, Caruso R, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget. 2014;5:2052–64. doi: 10.18632/oncotarget.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin Exp Metastasis. 2009;26:1005–12. doi: 10.1007/s10585-009-9290-7. [DOI] [PubMed] [Google Scholar]
- 25.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–51. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nafia I, Toulmonde M, Bortolotto D, Chaibi A, Bodet D, Rey C, et al. IDO Targeting in sarcoma:Biological and clinical implications. Front Immunol. 2020;11:274. doi: 10.3389/fimmu.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;6:874–81. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan:Much ado about IDO. Trends Immunol. 2003;5:242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Zhang F, Wang X, Liu K. The role of indoleamine 2, 3-dioxygenase 1 in regulating tumor microenvironment. Cancers (Basel) 2022;14:2756. doi: 10.3390/cancers14112756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meireson A, Devos M, Brochez L. IDO expression in cancer:Different compartment, different functionality? Front Immunol. 2020;11:531491. doi: 10.3389/fimmu.2020.531491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebau C, Baltzer AW, Schmidt S, Roesel C, Karreman C, Prisack JB, et al. Interleukin-12 and interleukin-18 induce indoleamine 2,3-dioxygenase (IDO) activity in human osteosarcoma cell lines independently from interferon-gamma. Anticancer Res. 2002;22:931–6. [PubMed] [Google Scholar]
- 32.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028):A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison DJ, Schwartz CL. Osteogenic sarcoma:Systemic chemotherapy options for localized disease. Curr Treat Options Oncol. 2017;18:24. doi: 10.1007/s11864-017-0464-2. [DOI] [PubMed] [Google Scholar]
- 34.Toda Y, Kohashi K, Yamada Y, Yoshimoto M, Ishihara S, Ito Y, et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients:Comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol. 2020;146:2607–20. doi: 10.1007/s00432-020-03242-6. [DOI] [PubMed] [Google Scholar]
- 35.Ligon JA, Choi W, Cojocaru G, Fu W, Hsiue EH, Oke TF, et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J Immunother Cancer. 2021;9:e001772. doi: 10.1136/jitc-2020-001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Q, Zuo J, Tian H, Huang C, Nice EC, Shi Z, et al. Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells. J Exp Clin Cancer Res. 2022;41:162. doi: 10.1186/s13046-022-02372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


