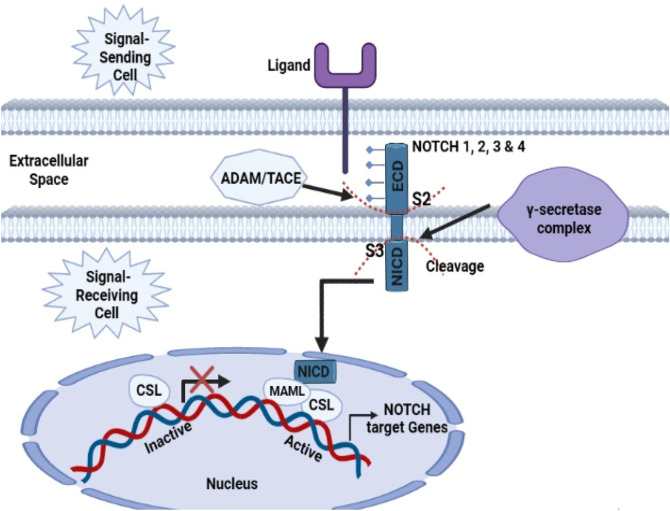
FIGURE 3.
Activation of the primary NOTCH1 signal. When a ligand is bound, two subsequent proteolytic cleavages start (S2 and S3). The initial cleavage at the S2 location of the extracellular domain is mediated by the ADAM/TACE proteinase. Accessibility to the γ-secretase complex, which is in charge of the subsequent proteolytic cleavage at the S3 site inside the transmembrane region, is made possible by the S2 cleavage. When the NICD domain is freed, it moves to the nucleus and engages with the CSL transcription factor. The activation of mastermind-like protein (MAML) is facilitated by the establishment of a composite-binding interface by docking the NOTCH ankyrin repeat domain to the CSL protein. Through the displacement of corepressors and histone deacetylases and the recruitment of histone acetyltransferases, these processes change CSL from a transcriptional repressor to a promoter. As a result of MAML recruitment of further coactivators, the NOTCH target genes are expressed.