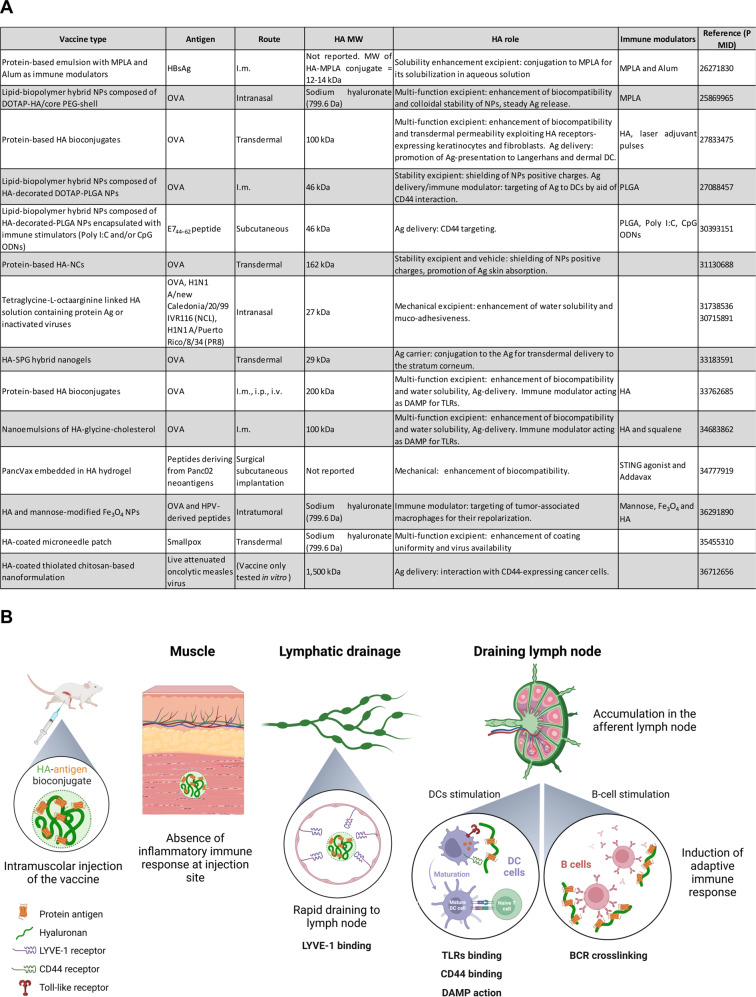
Fig. 1.
A The table summarizes HA-based vaccines validated preclinically from 2015 to 2023. MPLA = the detoxified derivative of lipopolysaccharide (LPS) isolated from the gram-negative Salmonella Minnesota R595 bacterial strain; HBsAg = recombinant hepatitis B surface antigen; NPs = nanoparticles; DOTAP = 1,2-dioleoyl-3-trimethylammonium-propane; PEG = poly(ethylene glycol); poly I:C = polyinosinic-polycytidylic acid; CpG ODN = CpG oligodeoxynucleotides; NCs = nanocapsules; SBG = β-glucan schizophyllan; STING = stimulator of interferon genes; HPV = human papilloma virus. B Graphical summary of the HA mechanism of action. Following intramuscular administration, HA-bioconjugates rapidly enter draining lymph nodes due to their small size and ability to bind the LYVE-1 receptor without inducing any inflammatory response at the injection site. In the draining lymph nodes, the bioconjugate is retained longer than soluble antigens, and HA binding to TLRs and CD44 allows optimal interactions with APCs, inducing the maturation of these cells. Additionally, the repetitive display of multiple antigens bound to an HA chain facilitates the crosslinking of B-cell receptors (BCRs) and results in sustained activation of B cells. Created with BioRender.com