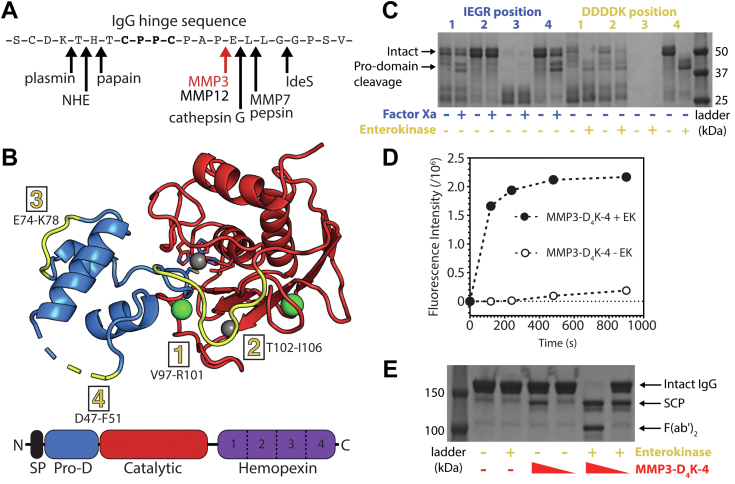
Figure 5.
Engineering and characterization of matrix metalloproteinase 3 (MMP3) for targeted endogenous human IgG cleavage. A, human and bacterial proteases previously shown to cleave within the hinge of human IgG, with the MMP3 cleavage site indicated in red. B, structural representation of MMP3 (PDB ID: 1SLM (64)). The prodomain (Pro-D, blue) and catalytic domain (catalytic, red) are shown in cartoon representation, while the signaling peptide (SP, black) and the hemopexin domain (hemopexin, violet) are not present in the published crystal structure. Calcium atoms and zinc atoms are shown in green and gray, respectively. The catalytic zinc is coordinated by three histidines within the catalytic domain and one cysteine within the pro-domain (shown as sticks). The four substitution sites for the factor Xa (IEGR) and enterokinase (DDDDK) cleavage sequences are highlighted in yellow and listed with the corresponding MMP3 residues. C, SDS-PAGE gel depicting the eight protease cleavage site insertion variants before and after activation with their respective external protease (factor Xa in blue and enterokinase in yellow). D MMP3-D4K-4 represents the variant with an enterokinase cleavage site substituted within position four of the MMP3 prodomain. This variant efficiently cleaves a fluorescent MMP3 peptide substrate after the prodomain is removed with enterokinase (filled circles), while the intact form containing the prodomain minimally cleaves the substrate (open circles). E, SDS-PAGE gel showing the cleavage of human IgG by MMP3-D4K-4 either with or without the prodomain at different relative concentrations (10% and 1% w/w) after 24 h at 37 °C. Presence or absence of a component in the reaction is represented by + and −, respectively. MMP3-D4K-4 cleaves the lower hinge of intact human IgG in a sequential manner, first producing a single cleavage product (SCP, in which half of the Fc is lost upon denaturation), then producing F(ab’)2 and Fc (not shown) after the second cleavage. Enterokinase does not detectably cleave human IgG (lane 3). IgG, immunoglobulin G.