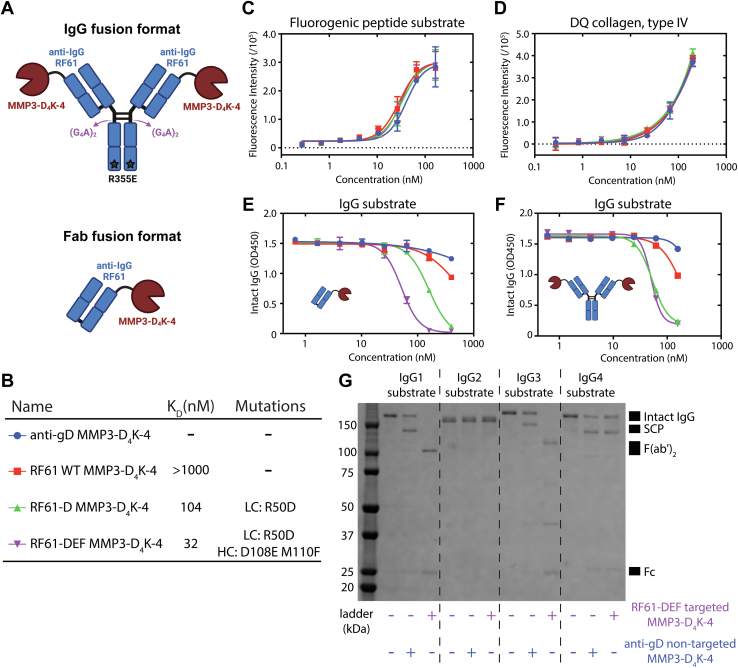
Figure 7.
Engineering a targeted protease for human IgG cleavage. A, cartoon representations of the IgG-MMP3-D4K-4 and the Fab-MMP3-D4K-4 fusion protein formats. B, summary of affinity and relevant mutations for the anti-IgG antibody affinity series tested in (C–F). Affinity was measured for the anti-IgG Fabs of RF61 variants against human IgG1 Fc using SPR (Fig. S8). C–F, in vitro cleavage assays measuring proteolytic activity of an anti-IgG affinity series of antibody-MMP3-D4K-4 fusion proteins against a fluorogenic MMP3 peptide substrate (C), DQ collagen, type IV (D), and human IgG1 (E and F). The Fab-MMP3-D4K-4 fusion format was used in (C–E), while the IgG-MMP3-D4K-4 fusion format was used in (F). Cleavage of the substrates in (C and D) generates fluorescent signal through dequenching of fluorophores. Cleavage of IgG in (E and F) is measured with ELISA. Error bars represent standard error values with n = 2 (C and D) and n = 3 (E and F). G, dependence of targeted and nontargeted IgG cleavage on human IgG subtypes. MMP3 does not cleave IgG2, while RF61 does not bind IgG4 (Fig. S9). Fab, fragment antigen binding; Fc, fragment crystallizable; IgG, immunoglobulin G; MMP, matrix metalloproteinase; SPR, surface plasmon resonance.