Table 3.
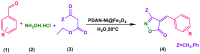
Benzaldehyde derivatives conversion to isoxazole-5(4H)-ones in the existence of PDAN-Ni@Fe3O4.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Productb | code | Time (min) | Yield %b | M.P (°C) |
Ref. | |
| Observed | Reported | ||||||
| 1 | 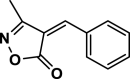 |
4a | 60 | 90 | 140–142 | 140–142 | [43] |
| 2 | 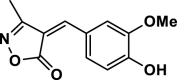 |
4 b | 25 | 95 | 214–216 | 211–214 | [43] |
| 3 | 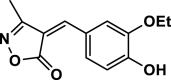 |
4c | 35 | 92 | 133–135 | 135–138 | [44] |
| 4 | 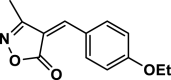 |
4 d | 40 | 96 | 140–142 | 139–140 | [44] |
| 5 | 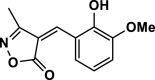 |
4e | 30 | 96 | 108–110 | 108–109 | [44] |
| 6 | 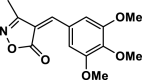 |
4f | 25 | 92 | 265–267 | 267–270 | [43] |
| 7 | 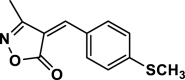 |
4 g | 45 | 95 | 171–173 | ||
| 8 | 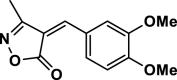 |
4 h | 30 | 93 | 135–137 | 135–136 | [45] |
| 9 | 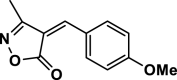 |
4i | 40 | 96 | 174–176 | 175–177 | [43] |
| 10 | 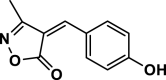 |
4j | 25 | 92 | 212–214 | 214–216 | [43] |
| 11 | 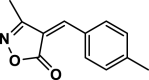 |
4 k | 30 | 90 | 135–137 | 135–136 | [43] |
| 12 | 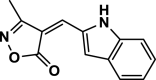 |
4 l | 60 | 93 | 241–243 | 242–244 | [45] |
| 13 | 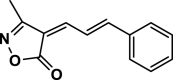 |
4 m | 80 | 90 | 174–176 | 173–174 | [46] |
| 14 | 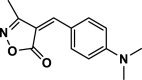 |
4n | 20 | 96 | 226–228 | 227–228 | [43] |
| 15 | 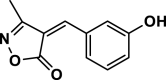 |
4o | 60 | 91 | 202–204 | 202–203 | [43] |
| 16 | 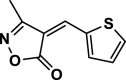 |
4p | 65 | 90 | 144–145 | 145–146 | [45] |
| 17 | 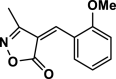 |
4q | 50 | 92 | 146–148 | 146–147 | [43] |
| 18 | 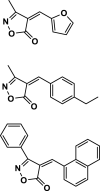 |
4r | 60 | 93 | 238–239 | 227–229 | [46] |
| 4s | 30 | 95 | 88–90 | ||||
| 4t | 60 | 90 | 174–176 | ||||
| 19 | 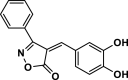 |
4u | 35 | 92 | 159–161 | 161–162 | [44] |
| 20 | 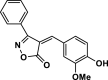 |
4v | 30 | 93 | 214–216 | 213–215 | [44] |
| 21 | 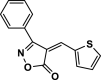 |
4w | 75 | 95 | 225–227 | 228–226 | [47] |
| 22 | 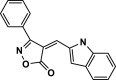 |
4× | 80 | 94 | 211–212 | 212–215 | [44] |
| 23 | 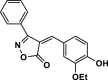 |
4 y | 40 | 96 | 149–151 | 150–152 | [44] |
| 24 | 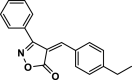 |
4z | 45 | 91 | 119–121 | 118–120 | [44] |
| 25 | 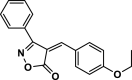 |
4aa | 35 | 95 | 130–131 | ||
| 26 | 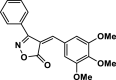 |
4 ab | 30 | 96 | 162–164 | 168–169 | [44] |
| 27 | 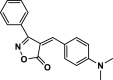 |
4ac | 25 | 91 | 196–198 | 194–196 | [47] |
| 28 | 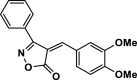 |
4ad | 40 | 90 | 168–170 | 168–170 | [17] |
| 29 | 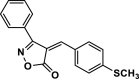 |
4ae | 45 | 92 | 184–186 | ||
| 30 | 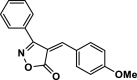 |
4af | 40 | 90 | 167–169 | 168–169 | [47] |
| 31 | 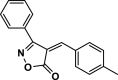 |
4 ag | 50 | 91 | 195–198 | 189–191 | [47] |
| 32 |  |
4ah | 120 | Not detected | |||
| 33 | 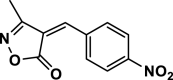 |
4ai | 120 | Not detected | |||
| 34 | 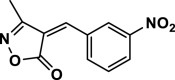 |
4aj | 120 | Not detected | |||
| 35 | 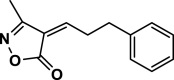 |
4ak | 120 | Not detected | |||
| 36 | 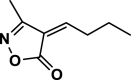 |
4 al | 120 | Not detected | |||
a Reaction conditions: aldehyde (1 mmol), β-ketoester (1 mmol), hydroxylamine hydrochloride (1 mmol), 5 mL water, and PDAN-Ni@Fe3O4 (0.04 g) at 50 °C.
Isolated yield.
