Abstract
Background
Drought is an understudied driver of infectious disease dynamics. Amidst the ongoing southwestern North American megadrought, California is experiencing the driest multi-decadal period since 800CE, exacerbated by temperature. We examined the influence of drought on coccidioidomycosis, an emerging infectious disease in the southwestern U.S.
Methods
We analyzed California census tract-level surveillance data from 2000–2020 using generalized additive models and distributed monthly lags on precipitation and temperature. We then developed an ensemble prediction algorithm of incident cases per census tract to estimate the counterfactual incidence that would have occurred in the absence of drought.
Results
An estimated excess of 1,467 and 2,649 drought-attributable cases were observed in California in the two years following the 2007–2009 and 2012–2015 droughts, respectively, more than offsetting declines experienced during drought. An interquartile range (IQR) increase in summer temperatures was associated with 2.02 (95% CI: 1.84, 2.22) times higher incidence in the following fall, and a one IQR increase in precipitation in the winter was associated with 1.45 (95% CI: 1.36, 1.55) times higher incidence in the fall. The effect of winter precipitation was 36% (95% CI: 25, 48%) stronger when preceded by two dry, rather than average, winters. Incidence in arid counties was most sensitive to precipitation fluctuations, while incidence in wetter counties was most sensitive to temperature.
Interpretation
In California, multiyear cycles of dry conditions followed by a wet winter amplifies transmission, especially in historically wetter areas. With anticipated increasing frequency of drought in southwestern U.S., continued expansion of coccidioidomycosis, along with more intense seasons, may be expected.
Keywords: coccidioidomycosis, grow and blow, drought, climate, precipitation, temperature, soil sterilization, California
Introduction
Coccidioidomycosis is an emerging infectious disease caused by inhalation of spores of the soil-dwelling fungal pathogen belonging to the Coccidioides genus, which can become airborne through wind erosion or soil disturbance [1]. Infection can lead to a primarily respiratory illness that can last months or may progress to a chronic state in 5–10% of individuals [1]. In California, age-adjusted incidence rates of coccidioidomycosis increased nearly 8-fold from 2000 to 2018, and more than tripled between 2014 and 2018 [2]. The highest incidence in California occurs in the hot and arid southern San Joaquin Valley, but the largest increases in incidence rates (>15-fold increase from 2000 to 2018) have been observed in cooler, wetter regions, along the coast and in the northern San Joaquin Valley [2]. Changing climatic factors that influence the distribution of suitable Coccidioides habitat may play a major role in the expansion and rise of coccidioidomycosis in California [3].
The emerging southwestern North American megadrought is intensifying, with 2000 to 2021 ranking as the driest 22-year period in California since at least 800CE [4]. Continued increases in drought frequency and severity may continue under anthropogenic warming [5]. California experienced one of its most severe droughts in recorded history between May 2012 and October 2015, receiving less precipitation in 2013 than in any previous calendar year since records began [5, 6]. The drought was exacerbated by record high temperatures [7] and was preceded by a less severe drought spanning March 2007 to November 2009 (Figure S1 on Supplement page 12) [8]. While public records from as early as 1980 show that statewide coccidioidomycosis incidence was lowest during drought and highest in years immediately following [9, 10], the causal effect of drought on coccidioidomycosis incidence has yet to be estimated.
Precipitation and temperature are factors driving drought occurrence that covary with the geographic range of Coccidioides spp. [3]. Periods of precipitation are thought to facilitate Coccidioides hyphal growth and sporulation [11, 12], and hot and dry periods cause the hyphae to autolyze, releasing infectious, heat-tolerant spores termed ‘arthroconidia’, and permitting dispersal of spores from desiccated soils [13–15]. Previous studies have reported associations between antecedent precipitation and transmission of C. posadasii in Arizona delayed by as much as 2–3 years [13], but no studies have examined whether antecedent conditions modify the influence of more recent meteorological effects in the months leading up to transmission. Here, we estimate immediate and delayed effects of temperature, precipitation, and drought on coccidioidomycosis incidence, arriving at new insights as to why California has observed substantial increases and geographic expansion in incidence over the past two decades.
Methods
Data
We obtained California Department of Public Health reportable disease surveillance data on coccidioidomycosis cases reported among California residents with estimated date of disease onset from April 1, 2000 – March 31, 2020. We matched 95% of patients to a census tract based on reported residence. For each census tract, we aggregated the total number of cases reported each month, assigning patients to the month indicated in the surveillance record for estimated disease onset. We also summarized information on total precipitation, average temperature, soil texture, impervious surface, and elevation summarized at the census tract and monthly scale (see Supplement pages 2–3 for details), and linked case to climate data by census tract and month.
To minimize exposure misclassification bias associated with assignment of cases to their census tract of residence, we restricted the study region to areas of known endemic transmission, limiting misclassification from travel-associated cases. We defined the study region to include 14 counties or sub-counties (herein after referred to as “counties”) where cumulative cases exceeded 500 over the study period and mean annual incidence rate exceeded 10 cases per 100,000 (Figure 1A; county names listed in Table S4 on Supplement page 10). Because the Sierra Nevada and San Emigdio-Tehachapi mountains produce strong climate gradients within the endemic counties of Kern, Fresno, Madera, Tulare, and Los Angeles, we split each into two “sub-counties” along a 500-meter elevation isocline and applied our inclusion criteria to each of the sub-counties. Both the areas on the low and high elevation side of the isocline in Kern County met the inclusion criteria, whereas only the lower elevation, western area of Fresno, Madera, and Tulare Counties, and the higher elevation, northern area of Los Angeles County, met the inclusion criteria.
Figure 1.
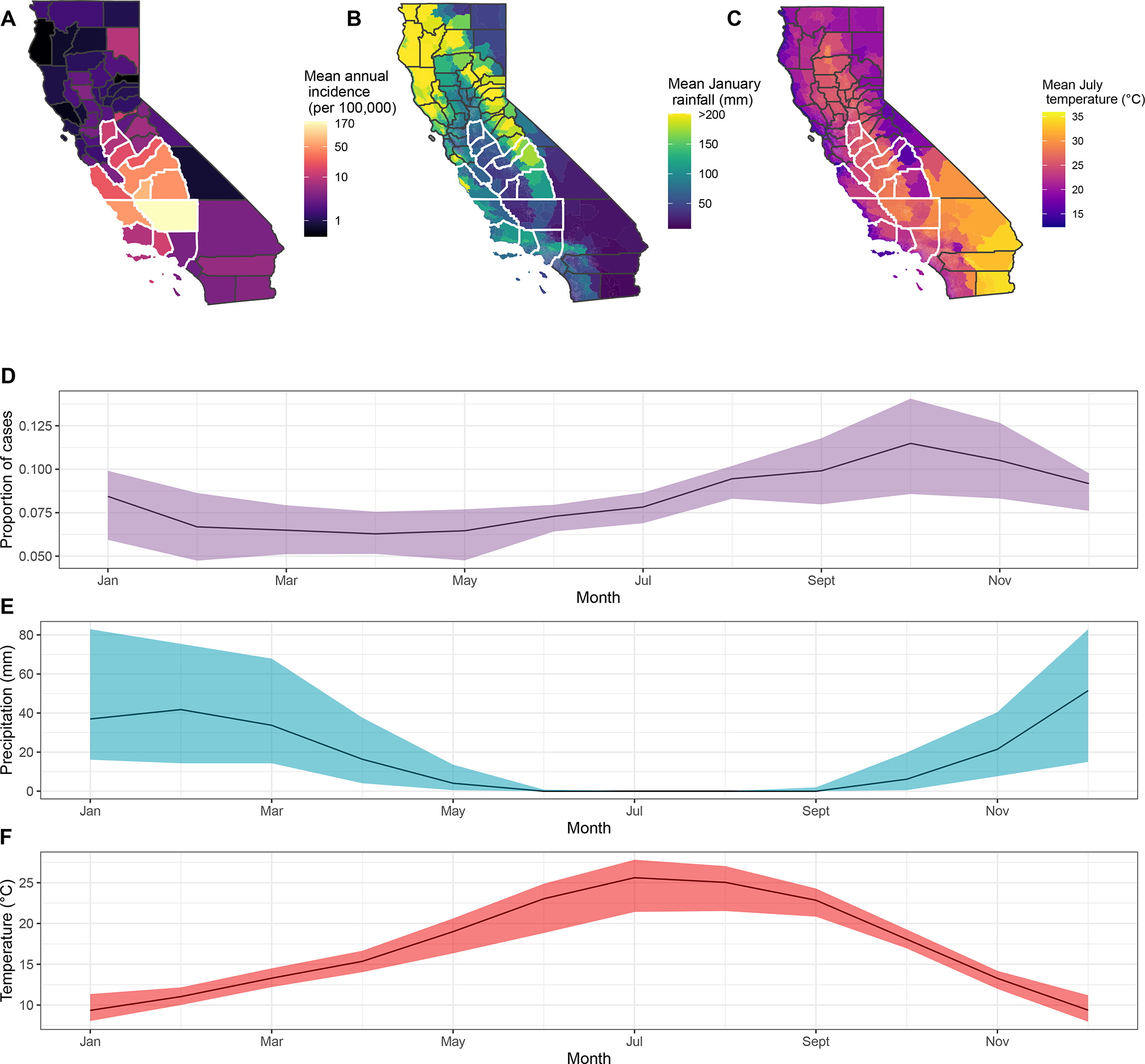
Average annual incidence of coccidioidomycosis (A) between 2000 and 2020. Mean total monthly precipitation (B) during January between 2000–2020. Average daily temperature (C) during July between 2000–2020. Counties outlined in white were included in analyses. Mean (dark line) and interquartile range (shaded area) of the proportion of annual cases (D) with an estimated date of onset per month, between 2000 and 2020 in the study region. Median (dark line) and interquartile range (shaded area) of monthly total precipitation (mm) (E) and mean daily temperature (degrees Celsius) (F) between 2000 and 2020 in the study region.
Distributed lag nonlinear regression models to estimate associations between climatic factors and incidence
Associations between coccidioidomycosis incidence and temperature and precipitation were estimated using a meta-analytic approach for estimating nonlinear, delayed effects across spatial locations [16]. We restricted this stage of our analysis to patients with an estimated disease onset between September through November—when most cases (33%) are typically reported in in California. As the effect of seasonal and lagged climatic factors may vary by season of disease onset [9,13], doing so enables more clear identification of the effect of climate in distinct seasons while improving comparability of our results with prior results. Incidence during September through November is strongly correlated with total incidence in a transmission year.
We first estimated county-specific associations of lagged monthly average temperature and total precipitation and monthly incidence using distributed-lag generalized additive models [16]. Model details are included in the Supplement, pages 3–4. We used monthly cases per census tract as the outcome variable and the log of each census tract’s population as an offset term so that model coefficients reflected logged incidence rate ratios (IRRs). The primary exposure variables were lagged total precipitation and mean temperature. These were modeled with natural cubic spline functions of smoothed three-month averages, with lags spanning 1 to 36 months prior to estimated date of onset. We included a natural cubic spline for soil type (percent sand) and year, the latter of which enables our exploration of shorter-term climate associations by controlling for reporting and other long-term secular trends not due to climate [17].
We pooled estimates of county-specific incidence rate ratios using fixed-effects meta-analysis [16]. We examined the overall shape of the spline relating precipitation and temperature to incidence at each of the 36 monthly lags. We identified the 25th and 75th percentiles of precipitation or temperature for the lagged months included in a 12-month cycle (e.g., a lag of one month corresponds to August - October), and calculated the incidence rate ratio per interquartile range (IQR) increase in temperature or precipitation, keeping other factors fixed. We assessed factors explaining heterogeneity in the temperature-incidence and precipitation-incidence relationships across counties by extending the fixed-effects meta-analysis to include meta-predictors of county-level information (e.g., median county total precipitation, median county temperature). To examine potential modification of the effect of recent (within the past year) precipitation by antecedent conditions (lagged by over one year), we multiplied the basis function for the cubic spline on precipitation by a binary indicator for whether or not the census tract had a drier than average winter in the two years prior to estimated date of disease onset, three years prior to date of onset, or both. All statistical analyses were conducted in R, version 3.3.1 (R Foundation for Statistical Computing), using the splines and dlnm package for fitting distributed lag generalized additive models and the mvmeta package for performing fixed-effect meta-regression modeling [16, 18].
Ensemble model to estimate changes in incidence attributable to drought in California
We estimated cases attributable to—or averted because of—major droughts in California between April 1, 2000 and March 31, 2020 using an ensemble modelling approach to predict incidence under counterfactual scenarios reflecting absence of drought [19, 20]. Because our target parameter was total estimated cases attributable to drought (rather than IRRs; see Table S1 on Supplement page 2), for this stage of the analysis we examined incidence throughout the entire year, rather than restricting incident cases to September through November. For each county, we modelled monthly cases per census tract using multiple candidate prediction algorithms that included: generalized linear models with increasing complexity with respect to variables and interaction terms; generalized additive models; and Random Forest [21] (see Supplement pages 4–7 for model details). Predictors varied by algorithm, but could include: season; year; soil texture; elevation; percent impervious surface; total lagged monthly precipitation; and lagged mean temperature. We calculated the sum of squared errors for each algorithm using leave-out-one-year cross-validation. We then generated an ensemble prediction by generating a weighted average of candidate model predictions where the weights were derived using non-negative least squares and were inversely associated with their out-of-sample prediction error.
Agricultural drought is defined by lack of soil moisture, attributable to low precipitation and/or high temperature [22]. We used a simple substitution estimator (G-computation [20]) to calculate the expected incident cases in census tract in month with observed covariates, and the primary exposure, , set to either observed or counterfactual values for lagged rainfall and temperature (equation 1). For counterfactual, “no-drought” scenario, we deterministically set any monthly average temperature higher than the historical average and any total monthly precipitation below the historical average to their monthly county-level means during the two droughts. We summed across specific time periods and across all census tracts in a county to estimate the number of expected cases in a county over a time period (equation 1).
| [1] |
The incident cases attributable to—or averted by—the drought, , were estimated as the difference between predicted cases under the observed conditions, , and those predicted under the counterfactual “average climate” scenario, (equation 2).
| [2] |
Because antecedent conditions as far back as three years may carry influence, we examined the attributable incidence separately for the 2007–2009 and 2012–2015 droughts in the two years following the end of each drought. Because seasonal incidence is lowest in March-April, we considered the change in incident cases “during drought” to include the period starting at the onset of the drought and extending until the end of the transmission season following the droughts. The two years post drought encompassed the full epidemiological seasons following the drought (e.g., April 1, 2010 – March 31, 2012; April 1, 2016 – March 31, 2018).
Results
Descriptive analyses
Between April 1, 2000 and March 31, 2020, there were 81,448 reported coccidioidomycosis cases throughout California. There were 62,002 (76.1%) cases among residents of the examined counties (Figure 1A), of which 33% of patients had an estimated onset in September – November (Figure 1D). Kern (170 cases per 100,000 individuals), Kings (104 cases per 100,000), and San Luis Obispo (43 cases per 100,000) counties had the highest average annual incidence rates (Figure 1A). Among the counties analyzed, precipitation and temperature exhibited strong seasonal patterns. Precipitation was lowest in summer and highest in winter (Figure 1E), while air temperature typically peaked in July (Figure 1F). Southern San Joaquin Valley counties had among the lowest precipitation and highest temperatures, while northern and coastal counties were wetter and cooler (Figure 1B; 1C).
Association between precipitation, temperature and fall (September – November) incidence across time and space
Effect of recent precipitation and temperature (1–4 months lag)
When analyzing data on cases with estimated onset from September through November, interquartile range (IQR) increases in precipitation in the one to four months prior to the estimated date of disease onset (i.e., during the typically dry summer and early fall), were negatively associated with fall incidence (IRRs by lag: 1 month prior: 0.87, 95% CI: 0.80, 0.94; 2 prior: 0.89, 95% CI: 0.85, 0.94; 3 prior: 0.95, 95% CI: 0.92, 0.98; 4 prior: 0.87, 95% CI: 0.81, 0.94; Figure 2A; Table S3 on Supplement page 9). Increasing precipitation in the month prior to estimated disease onset from the 25th percentile to the 75th percentile was associated with a 13% reduction in incidence rates in September – November. Exposure-response relationships for lags are shown in Figures S2A–C and Figure S3 on Supplement pages 13–14.
Figure 2.
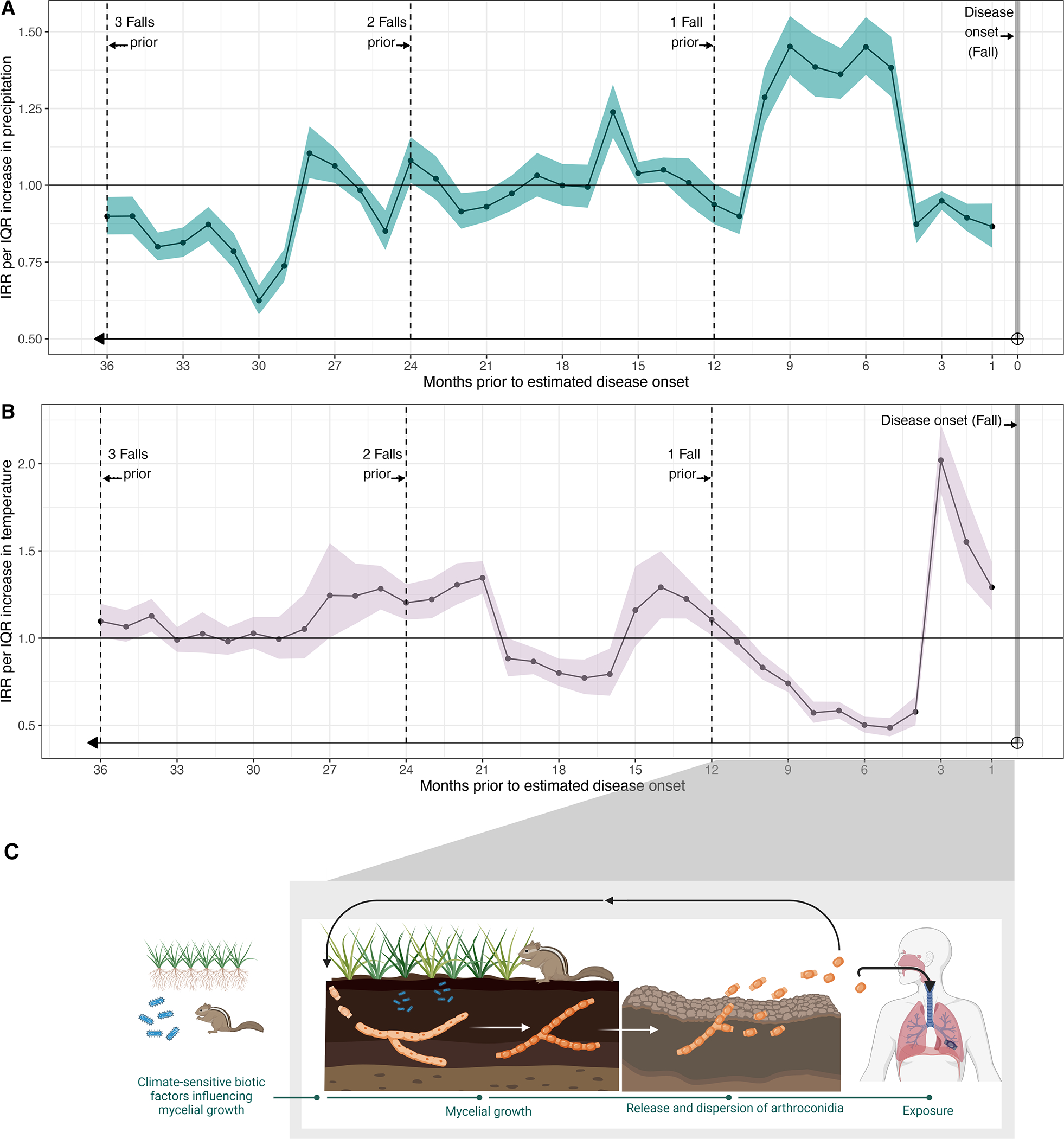
Results of distributed lag, generalized additive model testing the association between fall (September – November) incidence rates and lagged meteorological variables. Incidence rate ratios (IRRs) express the effect of an interquartile range (IQR) increase in precipitation (A) or temperature (B) in months prior to the estimated date of disease onset, with confidence intervals shown by shading. The horizontal line at one indicates null association (IRR=1). Panel C displays the saprobic lifecycle of Coccidioides and maps the hypothesized grow and blow cycle to the intra-annual wet-dry patterns. Inter-annual influences affecting mycelial growth might include biota, small mammals, and soil dwelling microbial competitors (in blue). These factors may be influenced by climate across inter-annual time scales
Average daily mean temperature in the one to three months prior to estimated disease onset, during the typically hot summer months, was positively associated with coccidioidomycosis incidence (IRRs by lag: 1 month prior: 1.29, 95% CI: 1.16, 1.44; 2 prior: 1.55, 95% CI: 1.32, 1.82; 3 prior: 2.02, 95% CI: 1.84, 2.22; Figure 2B, Table S3 on Supplement page 9). The exposure-response relationship at a 3-month lag (Figure S2D on Supplement page 13) showed that fall incidence increased with increasing summer temperature monotonically, with no apparent maximum beyond which temperatures are too hot. Exposure-response relationships for all lags are shown in Figures S2 D–F and Figure S4 (Supplement pages 13 and 15).
Effect of lagged precipitation and temperature (5–10 months lag)
Positive, significant pooled associations were detected between precipitation lagged 5–10 months and coccidioidomycosis incidence during September through November. The association peaked for precipitation in the winter prior to estimated date of disease onset (i.e., precipitation lagged 9 months; Figure 2A; Table S3 in Supplement page 9). An increase of total monthly winter precipitation from the 25th percentile (27.1 mm) to the 75th percentile (73.2 mm) in the 9 months preceding disease onset was associated with a 45% (IRR:1.45, 95% CI: 1.36, 1.55) increase in coccidioidomycosis incidence in the fall. Pooled exposure–response relationships for both winter and spring precipitation showed a unimodal response whereby an increase in coccidioidomycosis incidence was observed with incremental increases in precipitation until an optimal value was achieved (around 40–65 mm during spring, 80–105 mm during winter; Figure S2B, S2C, Supplement page 13), after which additional precipitation was associated with lower incidence as compared to the optimal.
Higher temperatures in the winter and spring prior to estimated date of disease onset (i.e., 5–10 months prior) were associated with suppressed incidence. In pooled analyses, an increase of one IQR in average monthly temperature in the winter prior to estimated date of disease onset (from 9.5°C to 12°C) was associated with a 26% (IRR: 0.74, 95% CI: 0.69, 79) decrease in incidence rates in the fall.
Antecedent precipitation and temperature (>12 months lag)
While total monthly precipitation in the winter immediately prior to estimated date of disease onset was positively associated with incidence, precipitation in the winters two and three years prior to estimated date of disease onset were negatively associated with incidence (Figure 2A). For temperature, warmer summers and cooler springs occurring two to three years prior to disease onset were associated with higher incidence, with a dampening of this association seen as the lag increased (Figure 2B).
Antecedent conditions modified the effect of more recent meteorological conditions. Low precipitation in the winters 2–3 years prior amplified the positive association between precipitation in the most recent winter and coccidioidomycosis. When following a year with low winter precipitation (i.e., a year with winter precipitation falling below the 50th percentile), an IQR increase in current year winter precipitation was associated with an IRR 1.19 (95% CI: 1.10, 1.30) times larger than the IRR when the same IQR increase in current year winter precipitation was experienced following a year with high winter precipitation (i.e., a year with winter precipitation above the 50th percentile). When following two years of below median precipitation, the effect of a one IQR increase in current year winter precipitation was 1.36 (95% CI: 1.25, 1.48) times the effect of the same increase in winter precipitation following two years in which precipitation for both years was not drier than average.
Heterogeneity in effects by precipitation and temperature gradients
In multivariate meta-regression models, we found that median winter precipitation in counties explained a significant amount of heterogeneity in county-specific IRRs representing the effect of winter precipitation on incidence (Figure 3A). The effect of a one IQR increase in winter precipitation (from 27 mm to 73 mm) was most pronounced among counties with low median monthly winter precipitation (Figure 3A). For instance, in western Kern, which experiences only 22.7 mm of total precipitation in a typical winter month, an increase from 27 to 73 mm of precipitation was associated with an IRR of 1.67 (95% CI: 1.42, 1.96). Precipitation in the wettest counties, such as Monterey County, which typically receives 70 mm of precipitation in a single winter month, had a non-significant negative effect on incidence. Thus, spatial variation in winter precipitation drove heterogeneity in the delayed effect of precipitation on coccidioidomycosis incidence, with dry counties most sensitive to fluctuations.
Figure 3.
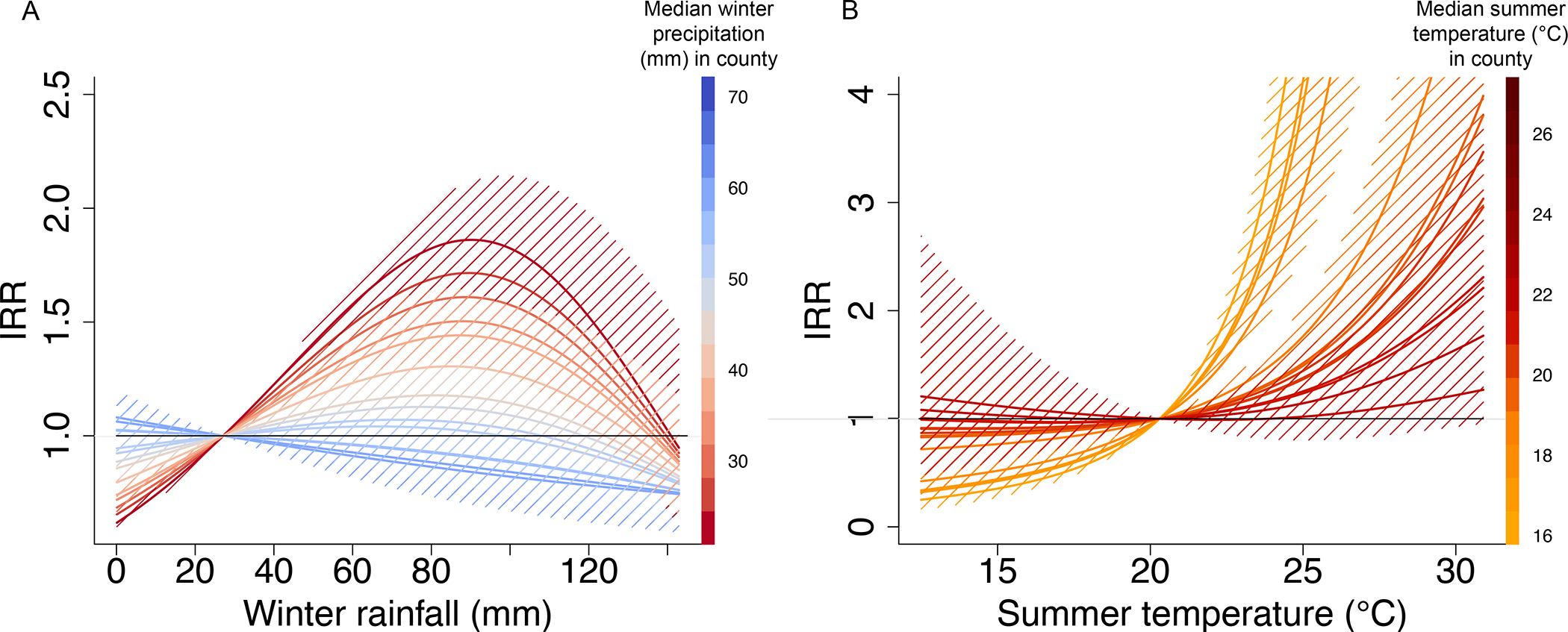
Increases in winter precipitation or summer temperature had the greatest effect in regions where rain was scarce or temperatures were low, respectively. (A) Estimated exposure-response relationships expressed as incidence rate ratios (IRR; colored lines) corresponding to the effect of changing winter precipitation and (B) summer temperature from a reference level to the value shown. The reference level for the IRR is the 25th percentile for the study region (for which IRR=1). Each line indicates an exposure-response relationship expressed as the incidence rate ratio for a given temperature or precipitation value compared to the incidence rate at the 25th percentile condition for a given county, based on the county’s median winter precipitation (A) or median mean summer temperature (B). Dashed regions around the solid lines indicate 95% confidence intervals. IRR = incidence rate ratio
Similarly, spatial variation in median summer temperature drove heterogeneity in the delayed effect of temperature on coccidioidomycosis incidence, with cooler counties most sensitive to fluctuations (Figure 3B). The effect of a one IQR increase in summer temperature (from 20.3°C to 25.8°C) was most pronounced among counties where the median summer temperature was coolest. For instance, in Monterey County, which experienced a mean monthly temperature of 16.2°C in a typical summer month, an increase from 20.3°C to 25.8°C in temperature was associated with an IRR of 12.7 (95% CI: 3.07, 53.3). An increase in mean summer temperature in the hottest 4 of 14 counties, such as western Kern County, which typically experiences a mean month temperature of 27.0°C, was non-significant.
Coccidioidomycosis incidence attributable to drought in California (ensemble models)
Ensemble models explained over 90% of the variation in coccidioidomycosis incidence in the study region (Figure 4A). In 10 of the 14 counties examined, the 2012–2015 drought was associated with lower than expected cases during the drought, followed by higher than expected cases in the two years following the drought (Figure 5 for relative changes, Table S4 and Figure S5, Supplement pages 10 and 16, for absolute changes). Due to the importance of winter precipitation on incidence in the following fall, the aversion of cases due to drought continued past the technical end of the drought, lasting until the end of the coccidioidomycosis transmission season (March 31). Across the study region and period, we estimated that drought averted 2,323 cases between May 1, 2012 – March 31, 2016, and caused 2,649 excess cases between April 1, 2016 – March 31, 2018 (Figure 4C; county-specific model fits shown in Figures S6–19 on Supplement pages 17–23).
Figure 4.
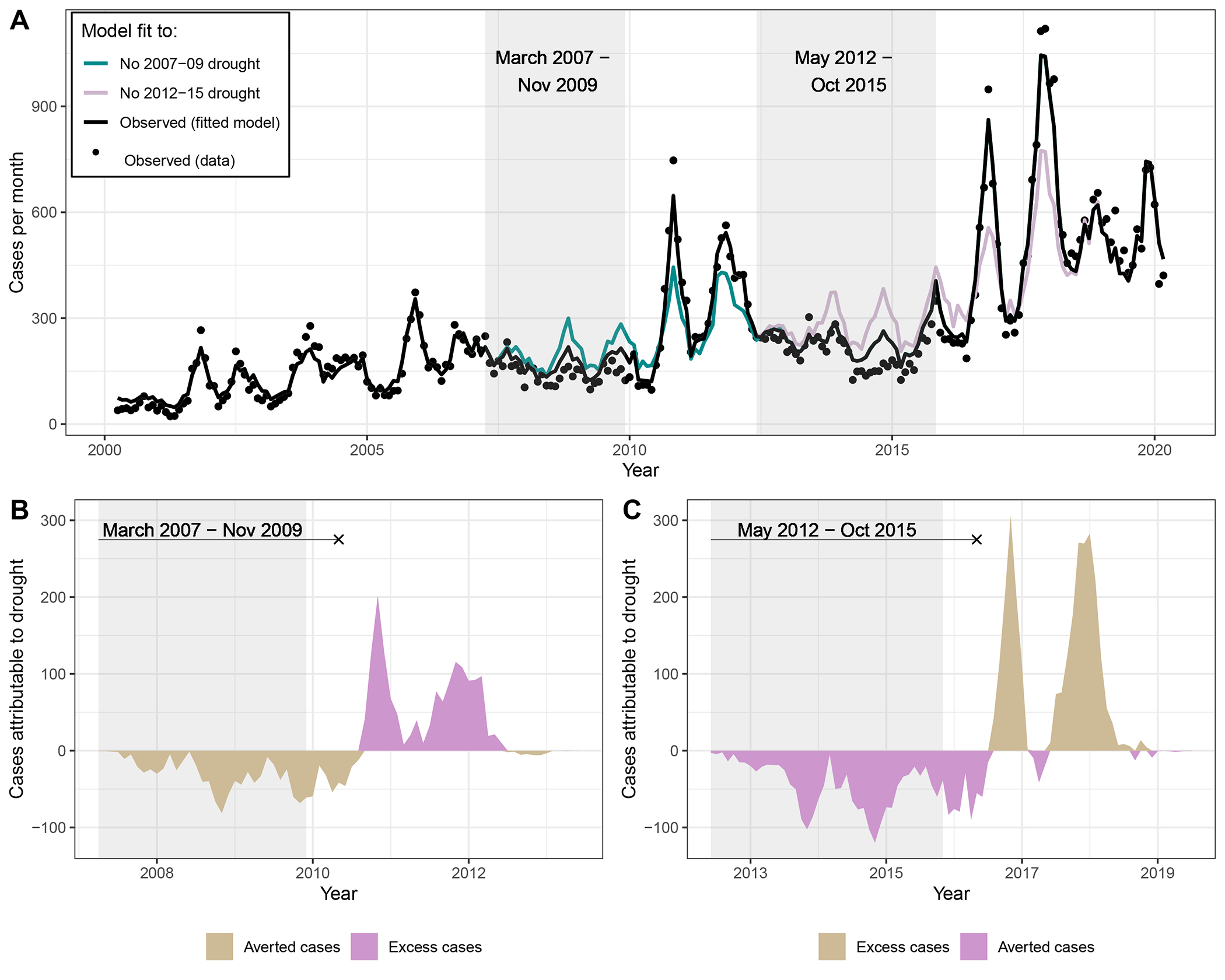
Droughts were associated with reduced incidence during the drought, and excess incidence following the drought. (A) Observed incidence (black dots) by month within the study region. Black line is the model fit under the observed environmental conditions. Color lines represent the expected incidence under the counterfactual intervention if the 2007–09 drought did not occur (cyan) or the 2012–15 drought did not occur (pink). Counterfactual scenarios were generated by setting temperatures observed to be higher than historical averages, and precipitation values observed to be below historical averages, deterministically to their average values. Gray boxes indicate the drought period. (B and C) Difference between expected cases and counterfactual cases if the 2007–09 (B) and the 2012–15 (C) droughts had not occurred, respectively. In B and C, the line symbol “——x” indicates the period that encompasses the drought and lasts until the end of the transmission season (March 31).
Figure 5.
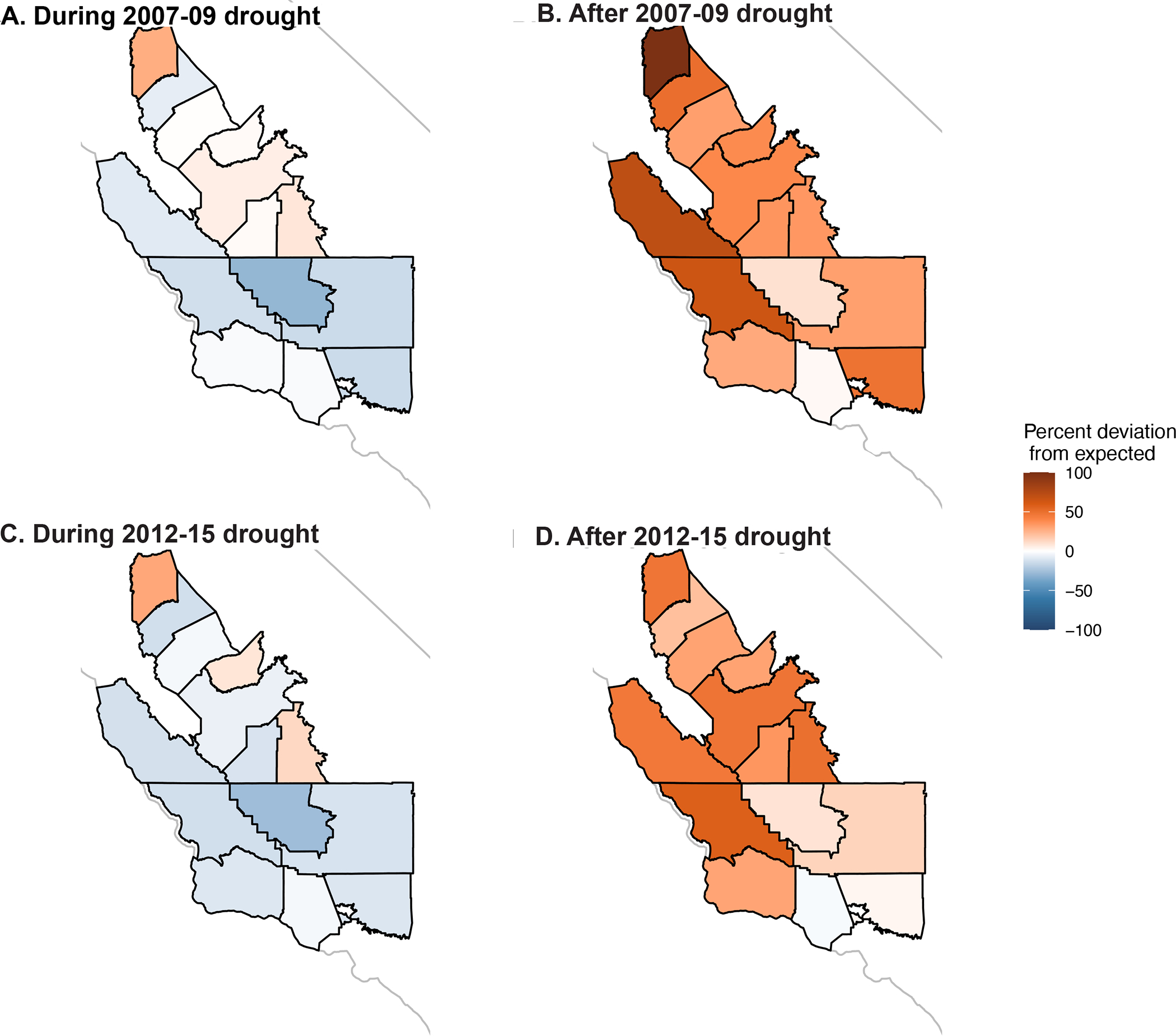
Estimated percent deviation in observed incident cases compared to the number expected in the absence of drought during (A and C) and in the two years following (B and D) the 2007–09 (A, B) and 2012–15 (C, D) droughts across the 14 counties in the study region. California state outline shown in light gray.
The shorter 2007–2009 drought followed similar patterns as the 2012–2015 drought but was associated with fewer averted cases during the drought. Across all counties examined, this drought was associated with 1,234 fewer cases between March 1, 2007 – March 31, 2010, and 1,467 excess cases between April 1, 2010 – March 31, 2012 in the study region (Figure 4B, Table S4 in Supplement page 10). For both droughts, >85% of estimated excess cases occurred during September – November, and nearly all occurred during August – November.
Kern County west of the Sierras has the highest incidence rates in California and is among the hottest and driest regions (Figure 1A–C). For both droughts, the decline in cases during the drought was most prominent in western Kern County and least pronounced in the counties in the northern San Joaquin Valley, while the increase following the drought was most prominent among the coastal counties and those in the northern San Joaquin Valley (Figure 5; Figure S5 on Supplement page 16). Over the 47 months spanning May 1, 2012 – March 31, 2016 drought period, 3,390 cases of coccidioidomycosis were reported among residents of western Kern County. We estimated that drought conditions were associated with an estimated 29.4% reduction from expected (counterfactual) incidence over May 1, 2012 – March 31, 2016 in Kern County and a 9.6% increase from expected incidence in the 24 months following the drought in Kern County. In comparison, we estimated that drought conditions were associated with an estimated 13% reduction in cases in the wetter San Luis Obispo County, which lies just west of Kern County along the coast, and an increase of 65% after the drought.
Discussion
We found that drought displaces coccidioidomycosis transmission, suppressing incidence in years characterized by drought conditions but amplifying incidence and seasonal peaks in the two years following. This demonstrates a previously unknown linkage between drought and transmission of an infectious disease. We found that incidence increased following wetter than average winters and hotter than average summers, and the magnitude of these effects was mediated by the underlying average climate regime of the region. The results provide evidence for inclusion of drought monitoring and seasonal climate forecasts in coccidioidomycosis surveillance and prediction.
The “grow and blow” hypothesis is among the most widely accepted mechanistic theories linking coccidioidomycosis transmission to climate conditions [13, 14, 23, 24], and hypotheses that coccidioidomycosis transmission is highest following wet periods post drought have circulated for at least three decades [10]. Our findings support and enhance our understanding of this hypothesis (Figure 2C; Table S5 in Supplement page 11) by suggesting that, within a transmission season, hyphal growth for C. immitis may be most important during winter and spring months when there is adequate rainfall to promote growth [12]. Meanwhile, higher temperatures and lower precipitation during summer and fall was associated with increased incidence, suggesting that lysis of hyphae into spores and wind dispersion of spores is most important in the summer and fall. Furthermore, we found that increasing summer temperatures were associated with especially pronounced relative increase in incidences among cooler counties as compared to already hot counties while increases in winter precipitation were associated with strongly increased incidence in very dry counties. This finding, along with our estimates of drought-attributable cases across counties, suggests the presence of “limiting factors” in the lifecycle of Coccidioides that vary by region. In arid regions, limited precipitation may restrict growth. In contrast, in cooler and wetter regions, the limiting factors may be insufficient heat to lyse the mycelia into individual arthroconidia and/or desiccate the soil to facilitate dust emissions, or excessive moisture for growth. This may explain why incidence rates have increased most dramatically in wetter and cooler counties, like the central coast counties, compared to the arid southern San Joaquin Valley counties [2].
Associations detected between coccidioidomycosis incidence and climatic conditions that occurred more than a year prior may involve the influence of upstream factors on pathogen proliferation, such as nutrient availability and presence of other soil microbes (Figure 2C; Table S5 on Supplement page 11). Both our ensemble model and regression results suggested that an increase in the mycelial growth that occurs during wet periods is induced by prior dry conditions, supporting hypotheses from as early as 1994 that abundant rains post drought increases transmission [10]. There are several hypotheses that may explain these acyclical inter-annual patterns. First, the “soil sterilization” hypothesis posits that extreme hot or dry periods may suppress the relative fitness of microbial competitors in the soil [13, 24], allowing Coccidioides populations to grow uninhibited by competition when more favorable conditions (i.e., moisture) returns. Coccidioides spp. are poor competitors for nutrients when compared with certain other soil fungi and bacteria [25], but are resilient and can survive climatological extremes [26]. Another hypothesis is that small mammals harbor inactive Coccidioides granulomas which transform into hyphae following host death, using the hosts’ keratin as nutrients [27]. Rodent death rate is highest during drought [28], which may lead to an accumulation of keratin in the soil.
This analysis was subject to exposure misclassification from assignment of cases to the month of their estimated date of onset, which was estimated either by the patient’s own report, or, absent this, as the date of specimen collection. Therefore, the lag between a change in a climatic factor and its associated change in disease incidence includes the incubation period for coccidioidomycosis, which varies between 7 and 21 days, and, for some patients, a lag between symptom onset and healthcare seeking, which is reported to be a median of 22 days [29], and a lag between healthcare seeking and testing. Exposure misclassification may bias the associations towards the null. Our focus on incident cases during September through November enabled us to parse out the influence of specific timing of wet and dry periods, but limited our ability to draw conclusions about how precipitation and temperature affect incidence at other times of the year. Our ensemble models examined incidence throughout the entire year, and the findings align qualitatively with regression results from September through November; both analyses found that incidence is suppressed during years with low precipitation, and a dry period before a wet period amplified the transmission-enhancing effect of the wet period. In modelling associations between climate variability and disease incidence, we do not control for factors that may lie on the causal pathway between temperature and incidence, such as near-surface winds or vegetation, even as they may play an important role in spore dispersal. The small mammal, endozoan-based lifecycle of Coccidioides introduced by Barker and Taylor [27] postulates that rainfall may enhance Coccidioides growth indirectly, by increasing food sources for small mammal populations that may be associated with the fungus. While indirect effects could contribute to delays in the effect of precipitation on transmission, our study is unable to distinguish direct from indirect effects. Finally, our results pertain to California, where C. immitis dominates, and may not be reliably extrapolated to other endemic areas where C. posadasii prevails. The geographic distributions of C. immitis and C. posadasii have little spatial overlap, and adaption to distinct environmental and climate factors within their habitats may have led to differences in thermotolerance or response to other climate stressors. For instance, the growth of C. immitis appears to be more limited than that of C. posadasii at high temperatures (37°C) [30].
With climate change, average winter precipitation is projected to see a modest increase in California [31], while precipitation in autumn and spring is projected to decrease [6, 32], which may enhance conditions favorable for Coccidioides growth and dispersion in the state. At the same time, anthropogenic climate change is expected, with medium-high confidence, to increase the duration, intensity, and frequency of temperature-driven drought in California and other western states [5, 33]. Accordingly, incidence may continue to expand into historically wetter and cooler regions, such as coastal counties and northern San Joaquin Valley counties in California. Beyond expansion, the seasonality of transmission, particularly following drought, may become more pronounced. Future analyses should consider how the relationships resolved in this work can inform the spatiotemporal distribution and the seasonality of coccidioidomycosis under anticipated climate regimes in the decades to come.
Supplementary Material
Research in Context.
Evidence before the study
Coccidioidomycosis is a major cause of community-acquired pneumonia in the southwestern United States caused by inhalation of fungal spores of the Coccidioides genus. Coccidioidomycosis has seen more than 8-fold increases in incidence and an expansion in geographic range over the past two decades, with the most dramatic increases occurring in areas that are historically wetter and cooler than the conditions thought to be most suitable for Coccidioides. Among the most concerning climatic changes recently observed in the southwestern U.S. is the increase in drought frequency and severity, a trend that may continue under anthropogenic warming. We searched for studies published in English up to February 21, 2022, with the terms “coccidioidomycosis AND drought” in PubMed and identified 10 results, published between 1994 and 2021. Two of these examined drought as a predictor of coccidioidomycosis in multivariate models. One hypothesized that the dramatic increases in case counts observed between 1991–1993 (2.7–10 times higher than average annual counts over the previous decade), was due to anomalously high precipitation in 1991–1992 that followed over 5 years of drought. None attempted to determine the causal effect of drought on cases. We then searched “coccidioidomycosis AND climate AND (precipitation OR rain OR temperature)” in PubMed, and identified 25 results. While prior studies generally support that alternating wet and dry periods enhance transmission, the role of these wet and dry periods—such as in the timing and duration for which they are associated with amplified risk, and the magnitude of this effect—are inconsistent by model structures, geographic foci, and approaches to disaggregate seasonal trends and account for lagged effects of climate. The prevailing use of traditional linear models in prior studies prevents generalization of results to geographies with differing climates.
Added value of this study
Here, we examine the relationships between temperature, precipitation and coccidioidomycosis incidence, including how the relationships vary across time periods and geographic areas, and the degree to which the effects of intra-annual climatic factors are modified by inter-annual climatic factors. We further estimated the causal effect of the 2007–2009 and 2012–2015 California droughts on coccidioidomycosis incidence across geographic areas, applying a non-parametric substitution-estimator (G-computation) approach to simultaneously describe space-varying, delayed, and nonlinear effects. We found that wet winters followed by hot, dry summers amplify transmission, with the largest amplification occurring when multi-year cycles of dry conditions are followed by a wet winter. Incidence in arid counties was most sensitive to precipitation fluctuations, while incidence in wetter coastal counties was most sensitive to temperature fluctuations. We found that drought temporarily displaces coccidioidomycosis transmission, suppressing cases in years characterized by drought conditions but amplifying cases in years immediately following drought. Nearly 2,650 excess cases of coccidioidomycosis were observed after the 2012–2015 drought attributable to the effect of drought on transmission. This more than offset the 2,323 cases of coccidioidomycosis estimated to be averted during the drought period.
Implications of all the available evidence
This study reveals a previously unknown relationship between drought and an emerging infectious disease, and offers the most comprehensive mechanistic examination to date of the role of meteorological factors and drought in the emergence and transmission of coccidioidomycosis in California. Our results are consistent with precipitation during winter and spring supporting C. immitis hyphal growth, and hot and dry summer and fall periods facilitating lysis of hyphae into singular spores that are amenable to wind dispersion. Our findings suggest the presence of “limiting factors” in the lifecycle of Coccidioides that vary by region. In arid regions, limited precipitation may restrict growth. In contrast, in cooler and wetter regions, the limiting factors may be insufficient heat to lyse the mycelia into individual arthroconidia and/or desiccate soils to facilitate dust emissions. Anthropogenic climate change is expected, with medium-high confidence, to increase the duration, intensity, and frequency of temperature-driven drought in California and other western states. Accordingly, coccidioidomycosis incidence may continue to expand into historically wetter and cooler regions, such as coastal counties and northern San Joaquin counties in California. Beyond expansion, more intense seasonal increases in disease may be apparent following drought.
Funding
This study was funded by National Institutes of Health grant no. R01AI148336. JRH was funded by National Institutes of Health grant no. F31AI152430. The funding source played no role in the writing of the manuscript or the decision to submit for publication.
Footnotes
Declaration of interests
None to declare.
Data availability statement
The R script used to conduct the data analysis is available in a publicly available GitHub repository (https://github.com/jrhead/ValleyFever_and_Drought). Human case data are protected health information (PHI) with access restricted to authorized California Department of Public Health (CDPH) staff. More complete human disease data can be obtained by submitting a formal request to the CDPH, Infectious Disease Branch, Surveillance and Statistics Section. All environmental predictors are publicly available.
References
- 1.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217–23. [DOI] [PubMed] [Google Scholar]
- 2.Cooksey GLS, Nguyen A, Vugia D, Jain S. Regional Analysis of Coccidioidomycosis Incidence—California, 2000–2018. Morbidity and Mortality Weekly Report. 2020;69(48):1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorris ME, Treseder KK, Zender CS, Randerson JT. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. GeoHealth. 2019;3(10):308–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams AP, Cook BI, Smerdon JE. Rapid intensification of the emerging southwestern North American megadrought in 2020–2021. Nature Climate Change. 2022. [Google Scholar]
- 5.Diffenbaugh NS, Swain DL, Touma D. Anthropogenic warming has increased drought risk in California. Proceedings of the National Academy of Sciences. 2015;112(13):3931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain DL, Tsiang M, Haugen M, Singh D, Charland A, Rajaratnam B, et al. The extraordinary California drought of 2013/2014: Character, context, and the role of climate change. Bull Am Meteorol Soc. 2014;95(9):S3–S7. [Google Scholar]
- 7.Mao Y, Nijssen B, Lettenmaier DP. Is climate change implicated in the 2013–2014 California drought? A hydrologic perspective. Geophysical Research Letters. 2015;42(8):2805–13. [Google Scholar]
- 8.United States Department of Agriculture. Unites States Drought Monitor 2020. [Available from: https://droughtmonitor.unl.edu/.
- 9.Centers for Disease Control and Prevention. Valley Fever Maps Atlanta, GA: CDC; 2019. [Available from: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/maps.html. [Google Scholar]
- 10.Pappagianis D. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin Infect Dis. 1994;19 Suppl 1:S14–8. [DOI] [PubMed] [Google Scholar]
- 11.Fisher FS, Bultman MW, Johnson SM, Pappagianis D, Zaborsky E. Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Annals of the New York Academy of Sciences. 2007;1111:47–72. [DOI] [PubMed] [Google Scholar]
- 12.Maddy KT. Ecological factors of the geographic distribution of Coccidioides immitis. Journal of the American Veterinary Medical Association. 1957;130(11):475–6. [PubMed] [Google Scholar]
- 13.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005;113(6):688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver EA, Kolivras KN. Investigating the Relationship Between Climate and Valley Fever (Coccidioidomycosis). EcoHealth. 2018;15(4):840–52. [DOI] [PubMed] [Google Scholar]
- 15.Gorris M, Cat L, Zender C, Treseder K, Randerson J. Coccidioidomycosis dynamics in relation to climate in the southwestern United States. GeoHealth. 2018;2(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Statistics in medicine. 2012;31(29):3821–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. International Journal of Epidemiology. 2013;42(4):1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 19.Polley E, Van Der Laan M. Super Learner in prediction. UC Berkeley Division of Biostatistics Working Paper Series. Working Paper 266, May 2010, http://biostats.bepress.com/ucbbiostat/paper266; 2010. [Google Scholar]
- 20.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Mathematical Modelling. 1986;7(9):1393–512. [Google Scholar]
- 21.Wright MN, Ziegler A. ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. Journal of Statistical Software. 2017;77.i01. [Google Scholar]
- 22.United States Geologic Service. California Drought: What is drought? : USGS; 2021. [Available from: https://ca.water.usgs.gov/california-drought/what-is-drought.html. [Google Scholar]
- 23.Coopersmith EJ, Bell JE, Benedict K, Shriber J, McCotter O, Cosh MH. Relating coccidioidomycosis (valley fever) incidence to soil moisture conditions. GeoHealth. 2017;1:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamerius JD, Comrie AC. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PloS one. 2011;6(6):e21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene DR, Koenig G, Fisher MC, Taylor JW. Soil isolation and molecular identification of Coccidioides immitis. Mycologia. 2000;92(3):406–10. [Google Scholar]
- 26.Friedman L, Smith CE, Pappagianis D, Berman R. Survival of Coccidioides immitis under controlled conditions of temperature and humidity. American Journal of Public Health and the Nations Health. 1956;46(10):1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor JW, Barker BM. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Medical Mycology. 2019;57(Supplement_1):S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prugh LR, Deguines N, Grinath JB, Suding KN, Bean WT, Stafford R, et al. Ecological winners and losers of extreme drought in California. Nature Climate Change. 2018;8(9):819–24. [Google Scholar]
- 29.Tsang CA, Anderson SM, Imholte SB, Erhart LM, Chen S, Park BJ, et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010;16(11):1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mead HL, Hamm PS, Shaffer IN, Teixeira MM, Wendel CS, Wiederhold NP, et al. Differential Thermotolerance Adaptation between Species of Coccidioides. Journal of fungi (Basel, Switzerland). 2020;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neelin JD, Langenbrunner B, Meyerson JE, Hall A, Berg N. California winter precipitation change under global warming in the Coupled Model Intercomparison Project phase 5 ensemble. Journal of Climate. 2013;26(17):6238–56. [Google Scholar]
- 32.Diffenbaugh NS, Giorgi F. Climate change hotspots in the CMIP5 global climate model ensemble. Climatic Change. 2012;114(3):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedsworth L, Cayan D, Franco G, Fisher L, Ziaja S. California’s Fourth Climate Change Assessment. 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R script used to conduct the data analysis is available in a publicly available GitHub repository (https://github.com/jrhead/ValleyFever_and_Drought). Human case data are protected health information (PHI) with access restricted to authorized California Department of Public Health (CDPH) staff. More complete human disease data can be obtained by submitting a formal request to the CDPH, Infectious Disease Branch, Surveillance and Statistics Section. All environmental predictors are publicly available.


