Fig. (1).
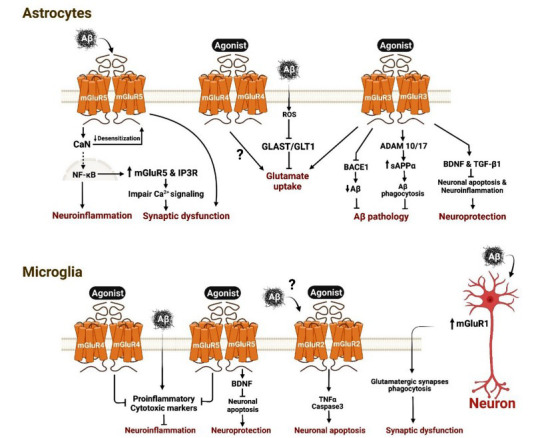
β-Amyloid (Aβ) neurotoxicity and neuroglial mGluRs. In astrocytes: β-Amyloid (Aβ) binds to mGluR5 and activates calcineurin (CaN) that facilitate the nuclear translocation of nuclear factor κB (NF-κB) and enhances the expression of neuroinflammatory markers. NF-κB increases expression of mGluR5 and inositol trisphosphate receptor 1 (IP3R1), leading to impaired Ca2+ signaling and synaptic dysfunction. CaN can also reduce mGluR5 desensitisation that contributes to synaptic dysfunction. Aβ increases the production of reactive oxygen species (ROS) that inhibits glutamate-aspartate transporter (GLAST) and Glutamate transporter 1 (GLT1), leading to a reduction in glutamate uptake. Agonist-dependent activation of mGluR3 and mGluR4 can enhance glutamate uptake, yet it remains debateable in the case of mGluR4. Agonist-dependent activation of mGluR3 inhibits β-secretase 1 (BACE1) that reduces the production of Aβ and activates α-secretases ADAM10 and 17 that increases the production of soluble amyloid precursor protein α (sAPPα), resulting in the phagocytosis of Aβ by astrocytes and microglia. Agonist-dependent activation of mGluR3 can also increase the production of brain derived neurotrophic factor (BDNF) and transforming growth factor β1 (TGF-β1) that reduces neuronal apoptosis and neuroinflammation and contribute to overall neuroprotection. In microglia: Aβ triggers the release of many proinflammatory and cytotoxic markers leading to neuroinflammation and neurotoxicity. Agonist-dependent activation of mGluR4 and mGluR5 reduces neuroinflammation. mGluR5 activation enhances the production of BDNF that supports neuroprotection by reducing apoptosis. Agonist or possibly Aβ-dependent activation of mGluR2 enhances the release of tissue necrosis factor α (TNFα) and activates caspase3 leading to neuronal apoptosis. Aβ increases the expression of mGluR1 in neurons that can act in paracrine manner to trigger the phagocytosis of glutamatergic synapses by microglia and contribute to synaptic dysfunction.
