Figure 1.
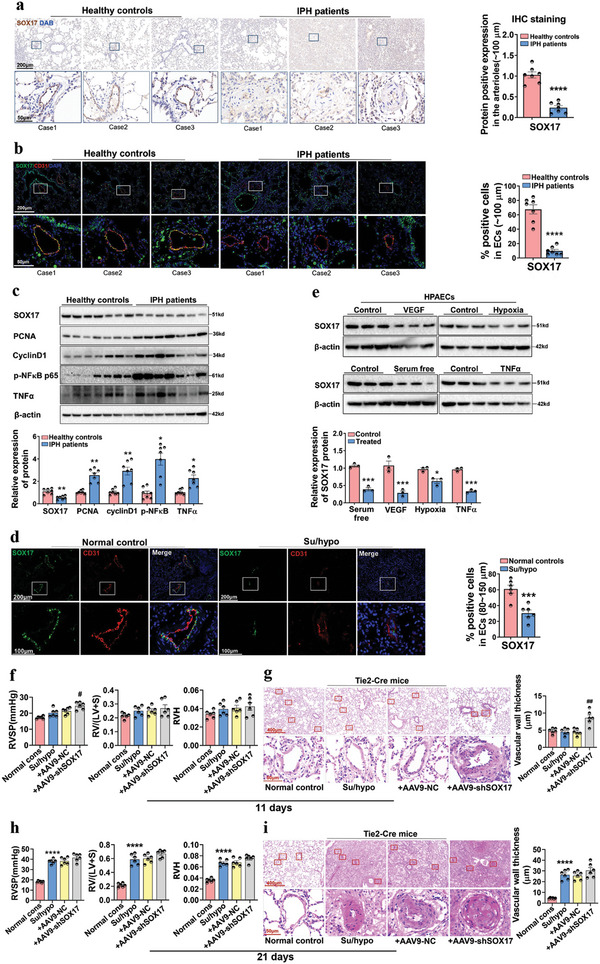
The expression of SOX17 is absent in the pulmonary arterial endothelium from IPH patients and in HPAECs treated by serum‐free medium, VEGF, hypoxia, and TNFα. a) IHC staining for the expression of SOX17 in the lung tissues of healthy donors and IPH patients, and the quantification of SOX17+ pulmonary arteries (≈100 µm). Values are mean fold‐changes compared to healthy controls, ****P < 0.0001 versus healthy controls, n = 7. b) Dual‐IF staining for the location and expression of SOX17 and CD31 in the lung tissues of healthy donors and IPH patients, and the quantification of SOX17+ and CD31+ pulmonary arteries (≈100 µm). Values are mean fold‐changes of healthy controls, ****P < 0.0001 versus healthy controls, n = 7. c) Western blotting assay for the expression of SOX17, PCNA, cyclinD1, p‐NFκB, and TNFα proteins in the lung homogenates from healthy controls and IPH patients, and the quantified data, *P < 0.05, **P < 0.01, ***P < 0.001 versus healthy controls, n = 7. d) Dual‐IF staining for the location and expression of SOX17 and CD31 in the lung tissues of mice treated with Su/hypo, ***P < 0.001 versus normal controls, n = 6. e) Western blotting assay for the protein expression of SOX17 in HPAECs treated with VEGF, serum‐free (HPAECs were seeded and allowed to attach for 24 h in the normal medium [ECM endothelial cells medium, supplemented with 5% FBS, 1% endothelial cell growth supplement, and 1% antibiotic solution]). The following day, the medium was removed, and the cells were cultured in serum‐free medium (ECM endothelial cells medium, supplemented with 0% FBS, 1% endothelial cell growth supplement, and 1% antibiotic solution), hypoxia, and TNFα, and the quantified data, *P < 0.05, ***P < 0.001 versus Control, n = 3. f) RVSP, right ventricular hypertrophy (RV/LV+S), RV weight/tibial length (RVH) in the Tie2‐Cre mice treated with AAV9‐shSOX17‐loxp (AAV9‐shSOX17) for 21 d and Su/hypo for another 11 d were examined, #P < 0.05 versus Su/hypo + AAV9‐NC‐loxp (AAV9‐NC), n = 6. g) HE staining for the vascular remodeling in the Tie2‐Cre mice, and the quantification of vascular wall thickness (80–150 µm), ##P < 0.01 versus Su/hypo + AAV9‐NC, n = 6. h) RVSP, RV/LV+S, RVH in the Tie2‐Cre mice treated with AAV9‐shSOX17 for 21 d and Su/hypo for another 21 d were examined, ****P < 0.0001 versus Normal controls, n = 6. i) HE staining for the vascular remodeling in the Tie2‐Cre mice, and the quantification of vascular wall thickness (80–150 µm), ****P < 0. 0001 versus Normal controls, n = 6.
