FIGURE 6.
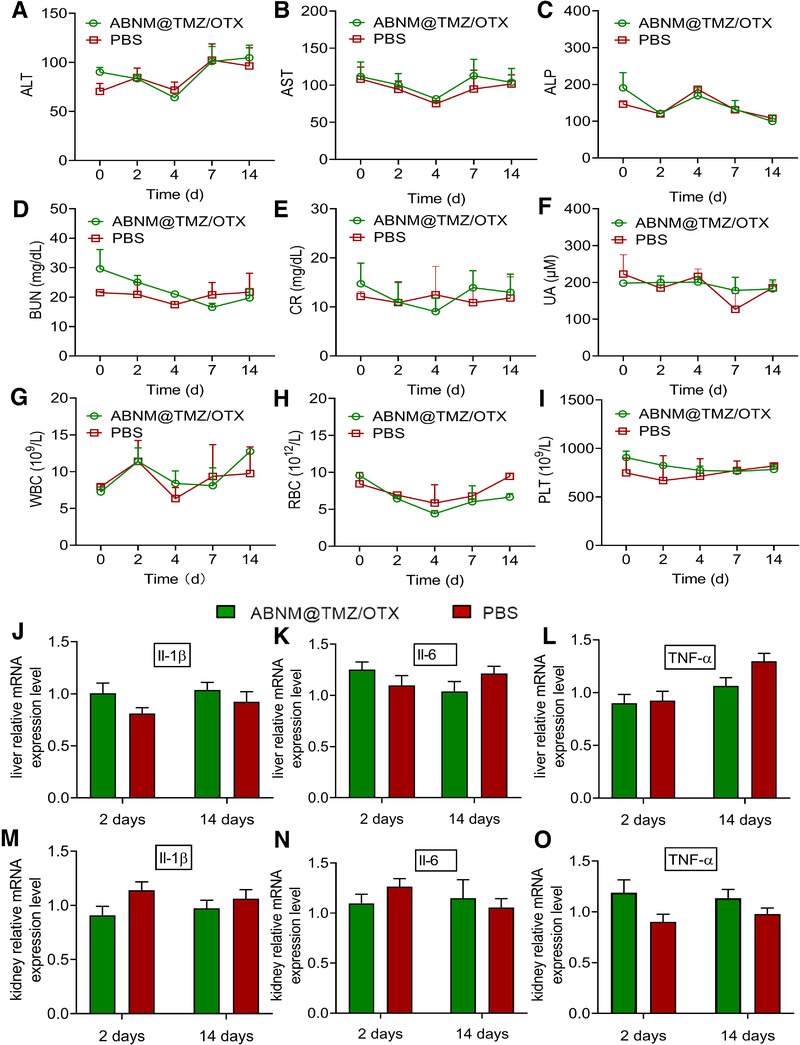
Biocompatibility evaluation of ABNM@TMZ/OTX. (A) Examination of plasma ALT, (B) AST, (C) ALP, (D) BUN, (E) CR, (F) UA contents after receiving ABNM@TMZ/OTX via tail vein (5 mg TMZ equiv./kg, 5 mg OTX equiv./kg). Routine blood examinations include (G) WBC counts, (H) RBC counts, and (I) PLT counts. Levels of pro‐inflammatory cytokines Il‐1β, Il‐6, and TNF‐α in liver (J–L) and kidney (M–O) were quantified after receiving ABNM@TMZ/OTX or PBS at days 2 and 14
