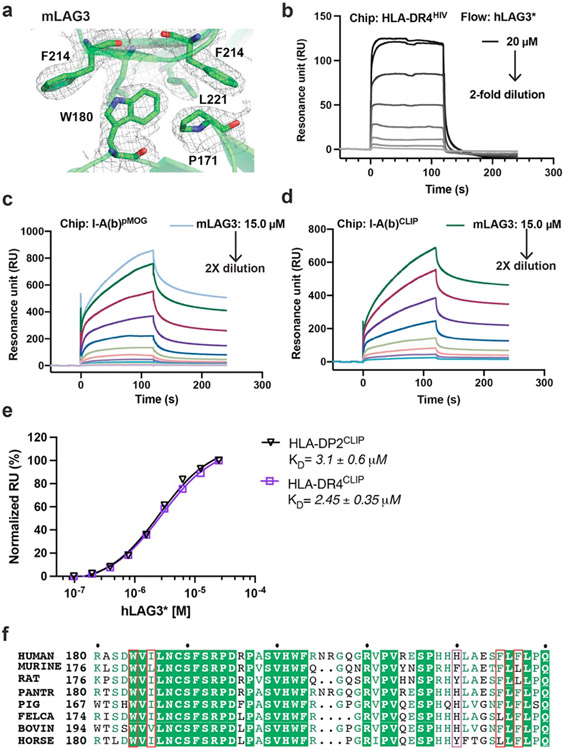
Extended Data Figure 3. LAG3 dimer interface and LAG3:MHCII binding analyses with SPR.
a, The 2Fo-Fc electron density map, contoured at 1.0 σ, surrounding the mLAG3 dimer interface residue Trp180. b, SPR analyses of hLAG3* binding to HLA-DR4HIV. Biotinylated HLA-DR4HIV was immobilized on a SA chip. hLAG3* protein dilutions starting from 20,000 nM were injected in turn. c-d, SPR sensorgram of recombinant mLAG3 flowed over an SA-chip immobilized with I-A(b)MOG (c) or I-A(b)CLIP (d). e, SPR analyses of LAG3 binding affinity binding to human MHCII allomorphs. MHCII proteins bound to CLIP peptides were biotinylated and immobilized on an SA chip and hLAG3* proteins were flowed over the chip. The curves were then fitted to determine the KD. Mean KD and S.D was from two independent replicates. f, Sequence alignment of the D2 dimer interface region from multiple LAG3 orthologs. Residues forming dimer contacts are outlined in red, conserved residues are highlighted in green, and biochemically similar residues are colored in green.