Abstract
Outcomes following heart transplantation remain suboptimal with acute and chronic rejection being major contributors to poor long-term survival. IL-6 is increasingly recognized as a critical pro-inflammatory cytokine involved in allograft injury and has been shown to play a key role in regulating the inflammatory and alloimmune responses following heart transplantation. Therapies that inhibit IL-6 signaling have emerged as promising strategies to prevent allograft rejection. Here, we review experimental and pre-clinical evidence that supports the potential use of IL-6 signaling blockade to improve outcomes in heart transplant recipients.
Keywords: basic (laboratory) research/science, cytokines/cytokine receptors, heart (allograft) function/dysfunction, heart transplantation/cardiology, immunosuppression/immune modulation, immunosuppressant–fusion proteins and monoclonal antibodies, rejection: acute, rejection: chronic, solid organ transplantation, translational research/science
1 |. INTRODUCTION
Inflammation and alloimmunity remain major barriers to the long-term survival of heart transplant recipients. The combined effects of early inflammation and immune activation contribute to a > 10% one-year incidence of acute cellular rejection (ACR),1 >20% incidence of antibody-mediated rejection (AMR) and de novo donor-specific allo-antibody (DSA) formation,2,3 and 47% incidence of cardiac allograft vasculopathy (CAV).4 Along with infection and malignancy, these complications result in median post-transplant survival of 12.5 years, with the risk of death or re-transplantation approaching 10% in the first year after transplantation.4
IL-6 is a pleiotropic cytokine that has recently emerged as a target for clinical intervention in numerous conditions including autoimmunity5–7 and immune-mediated injury following kidney transplantation.8,9 Importantly, IL-6 signaling has been shown to serve a critical role in the processes underlying the development of ischemia–reperfusion injury (IRI), ACR, AMR, and fibrosis (including CAV) following heart transplantation.10 While conventional immunosuppression has focused on controlling T cell mediated responses, it is increasingly recognized that acute inflammation, humoral and innate immunity also have deleterious effects on the heart allograft and its recipient. Together with compelling pre-clinical evidence, these findings highlight the therapeutic potential for harnessing IL-6 blockade to promote long-term graft and patient survival in clinical cardiac transplantation.
Currently, available agents for IL-6 signaling inhibition include monoclonal antibodies against the cytokine IL-6 or the IL-6 receptor (IL-6R) and Janus kinase inhibitors.11,12 Clinical trials in kidney transplantation have demonstrated promising results with the use of tocilizumab, a humanized monoclonal antibody against IL-6R, for desensitization and prevention/treatment of AMR8,13–16; tocilizumab is approved in multiple countries for the treatment of inflammatory/autoimmune diseases but is not yet approved for use in transplantation.17 Clazakizumab is an anti-IL-6 mAb that is not yet FDA-approved but has also demonstrated promising results for desensitization and treatment of late/chronic AMR in kidney transplant recipients9,18,19; a multicenter, randomized, and placebo-controlled clinical trial (IMAGINE) of clazakizumab in kidney transplant recipients with chronic AMR is currently underway (NCT03744910). A potential advantage of direct IL-6 (clazakizumab) over IL-6R blockade (tocilizumab) is the lack of rebound alloimmune response resulting from the accumulation of serum IL-6 that may occur with discontinuation of anti-IL-6R therapy; however, studies comparing the efficacy of IL-6 versus IL-6R blockade in transplantation have not yet been performed.
Here, we review the experimental and pre-clinical evidence in support of IL-6 signaling inhibition as a promising therapeutic option to prevent rejection following heart transplantation. We also discuss the clinical applications of IL-6 blockade for desensitization and the induction of immune tolerance in recipients of cardiac allografts.
2 |. ROLE OF IL-6 IN CARDIAC ALLOGRAFT INJURY
2.1 |. Acute inflammation/innate immunity
The detrimental effects of IL-6 begin prior to heart procurement, with marked upregulation of IL-6 in the setting of brain death resulting in significant intra-graft inflammation.20,21 Despite the increasing use of normothermic machine perfusion, static cold storage remains the most common preservation method in heart transplantation and further contributes to IL-6-related allograft injury in the form of IRI.22–26 Prolonged ischemic times, which exacerbate IRI, are known risk factors for primary graft dysfunction (PGD), acute and chronic rejection, CAV, and poorer 30-day survival.27–33 Following implantation, graft-derived IL-6 serves as an innate danger signal that promotes the activation of peripheral CD4+ and CD8+ effector T cells (Teff) through an antigen-independent mechanism.34
2.2 |. Adaptive immunity
Adaptive cellular and humoral immune responses are also tightly regulated by IL-6-dependent signaling. Myocardial biopsies and serum from heart transplant recipients demonstrate increased transcription of IL-6 mRNA and elevated peripheral IL-6 levels during acute rejection, which correlate with the severity of histologic cardiac allograft rejection.35–39
IL-6 promotes the expansion of the effector/memory CD8+ T cell populations by augmenting the expression of IL-2. Due to a shared reciprocal origin of Tregs and Th17 cells, IL-6 skews the lineage commitment of naïve CD4+ T cells toward the harmful Th17 effector phenotype and away from the potentially beneficial regulatory T cell (Treg: CD4+, CD25+, FoxP3+) phenotype.40,41 Th17 cells produce the cytokine IL-17, which promotes neutrophil proliferation and migration, endothelial cell activation, and fibroblast activation/proliferation, all of which contribute to acute and chronic allograft rejection.41,42 IL-17 creates a positive feedback loop with IL-6 by stimulating monocytes/macrophages, endothelial cells, and other cell types to produce pro-inflammatory cytokines, including IL-6 itself.43 In addition, graft-derived IL-6 amplifies allogeneic T cell responses that contribute to vascular rejection and allograft arteriosclerosis.44
The importance of AMR in cardiac transplantation is increasingly recognized due to its association with late allograft failure and CAV.45–47 IL-6 is central to nearly all aspects of humoral immunity, including T-follicular helper cell (Tfh) induction, germinal center formation, maturation of naïve B cells into plasmablasts/plasma cells, and production of high-affinity antibodies.48–51 The differentiation of naïve T cells into IL-21-producing Tfh cells is dependent on IL-6, which enables Tfh cells to progress through the germinal center and participate in antibody-mediated immunity. In this way, IL-6 is involved in coordinating both the T and B cell interactions responsible for robust adaptive alloimmune responses.52,53
2.3 |. Fibrosis/CAV
Fibrosis of the myocardial interstitium and coronary vasculature are pathologic components of chronic rejection.54 Allograft fibrosis is augmented by IL-6, which stimulates collagen production by fibroblasts, myofibroblast differentiation, and proliferation/activation of vascular smooth muscle and endothelial cells.55 Innate immune signals via toll-like receptors (TLR), IL-1 or tumor necrosis factor (TNF) receptors can induce IL-6 production in a variety of epithelial and endothelial cells.56 Importantly, IL-6 is capable of activating NK cells in long-term cardiac allografts and thereby contributes to the development of NK-mediated CAV.57
3 |. IL-6 BLOCKADE IN HEART TRANSPLANTATION
3.1 |. Experimental mouse models
Murine models provide substantial support for the use of IL-6 signaling inhibition in preventing/mitigating cardiac allograft rejection. For instance, BALB/c cardiac grafts transplanted into wild-type or IL-6-deficient C57BL/6 mice treated with costimulatory blockade (CTLA4-Ig) resulted in graft acceptance in the IL-6 deficient but not wild-type recipients.58 Mechanistic analyses revealed that the combined effect of costimulatory blockade and IL-6-deficiency limited Teff differentiation and promoted Treg migration into the grafts. More recently, perioperative anti-IL-6 mAb in combination with ATG induction was shown to prevent costimulatory blockade-resistant rejection, with increased intra-graft Tregts, prevention of CD8+ memory T cell formation, and reduced DSA formation.59 Another study showed that neutralization of IL-6 in CD4+ cell-mediated cardiac allograft rejection prolonged graft survival and was associated with decreased graft infiltrate and altered Th1 responses; in CD8+ cell-dominant graft rejection, neutralization of IL-6 delayed the onset of ACR and reduced graft T cell infiltrates.60
The role of IL-6 signaling inhibition in the prevention of AMR was recently investigated using a sensitized murine heart transplant model.61 Recipients underwent donor skin transplant for presensitization followed by heart transplantation and treatment with anti-IL-6R mAb. In recipients treated with anti-IL-6R mAb, there was a reduction in both circulating and intra-graft B cells along with reduced DSA production and complement activation, which was shown to attenuate allograft injury and improve survival compared to controls. The combined results from these studies have established the role of IL-6 signaling blockade in preventing/mitigating acute cellular and antibody-mediated rejection in murine heart transplant recipients.
Findings from mouse studies have also illustrated the role of IL-6 signaling blockade in preventing chronic rejection following heart transplantation. Induction of chronic cardiac allograft rejection through transient depletion of CD4+ cells revealed elevated intra-graft IL-6 expression; subsequently, treatment of CD4+ cell-depleted heart allograft recipients with anti-IL-6 mAb abrogated development of cardiomyocyte hypertrophy, graft fibrosis, and deterioration in graft function and thereby prevented graft failure due to chronic rejection.62 In another study, IRI was shown to exacerbate chronic allograft rejection in a mouse heart transplant model63; local delivery of IL-6-inhibiting nanoparticle therapy successfully abrogated IRI and reduced the development of chronic heart allograft rejection.
3.2 |. Pre-clinical non-human primate data
Our group recently achieved heart allograft tolerance in non-human primates (NHPs) for the first time by combining a mixed chimerism protocol with donor kidney co-transplantation.64 Prior to this, tolerance to kidney allografts had been achieved in MHC-mismatched NHPs using a mixed chimerism approach; however, application of the same protocol with isolated heart allografts consistently resulted in early rejection.64,65 Preliminary evidence suggests that kidney but not heart allografts are able to augment/activate host Tregs which are likely responsible for kidney-induced cardiac allograft tolerance.66 Given these findings and the recognition that co-transplantation of a donor kidney simply to achieve heart allograft tolerance in patients is untenable, we attempted to substitute therapies aimed at Treg expansion (including IL-6 signaling inhibition) in place of a co-transplanted donor kidney.
IL-6 signaling blockade and low-dose IL-2 have both been shown to preferentially expand Tregs.67 We hypothesized that combining IL-6 signaling blockade with low-dose IL-2 in a mixed chimerism NHP protocol would promote Treg expansion resulting in heart allograft tolerance in the absence of donor kidney co-transplantation. In preliminary studies, NHPs undergoing mixed chimerism that received combined anti-IL-6R therapy (tocilizumab) and low-dose IL-2 experienced significant Treg expansion with a marked increase in the percentage of peripheral Tregs compared to pre-transplant baseline. However, all treated recipients rejected their heart allografts due to ACR and AMR.68,69 Failure to achieve prolonged heart allograft survival by combining low-dose IL-2 with anti-IL-6R therapy may have resulted from IL-2-induced T cell alloreactivity, as IL-2 can promote either immune activation or down-regulation in a dose-dependent fashion with a narrow therapeutic window.67 Therefore, another preliminary group of NHP recipients was treated with anti-IL-6R mAb therapy alone as part of a mixed chimerism-based protocol. Despite less significant Treg expansion, most anti-IL-6R mAb treated recipients achieved long-term heart allograft survival off all immunosuppression70 (Figure 1).
FIGURE 1.
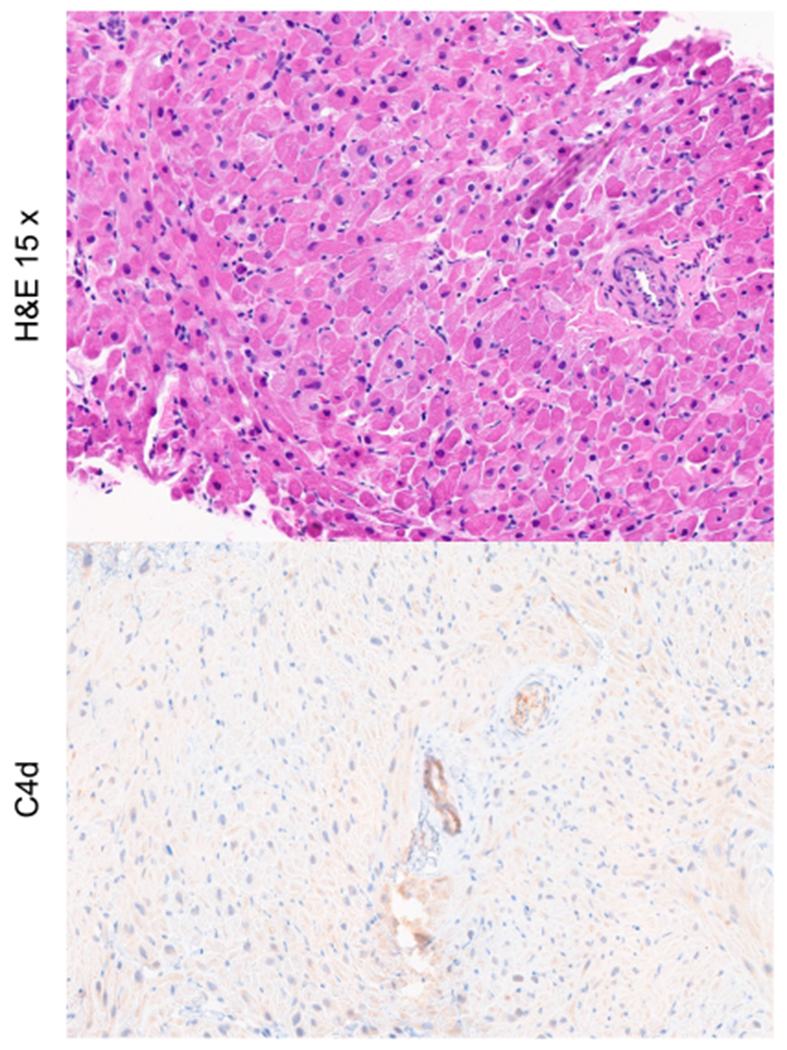
Heart allograft histology obtained >400 days post-transplant in NHP recipients that received anti-IL-6R mAb therapy as part of a mixed chimerism-based protocol, demonstrating no evidence of ACR or pathologic AMR.
Our ability to achieve long-term survival of some cardiac allografts in preliminary NHP studies using anti-IL-6R therapy despite a lack of significant Treg expansion suggests the contribution of mechanisms other than those purely regulatory in nature. Ongoing mechanistic studies will help to elucidate the mechanisms by which IL-6 signaling inhibition contributes to the long-term survival of cardiac allografts in this model. In the interim, these early results support the hypothesis that incorporating IL-6 signaling blockade into immunosuppressive therapies will improve outcomes in clinical cardiac transplantation.
3.3 |. Clinical applications
Based on experimental and pre-clinical evidence supporting the use of IL-6 signaling blockade to prevent heart allograft rejection, a randomized, multicenter phase II clinical trial investigating tocilizumab (FDA-approved, humanized anti-IL-6R mAb) as immunosuppressive therapy in heart transplantation was initiated in 2018 and is currently ongoing (NCT03644667; Targeting Inflammation and Alloimmunity in Heart Transplant Recipients with Tocilizumab). In this NIAID/ NIH-funded trial, 200 primary heart transplant recipients from over 14 centers are randomized 1:1 to standard triple-drug maintenance immunosuppression with placebo or monthly tocilizumab (8 mg/kg IV) for 6 months. Standard triple-drug immunosuppression consists of a calcineurin inhibitor (tacrolimus), an anti-proliferative agent (mycophenolate mofetil/enteric-coated mycophenolate sodium), and steroids (methylprednisolone/prednisone). Adults (aged 18 to 75 years) undergoing primary heart transplant with a negative virtual crossmatch, no additional organ/tissue transplant, and no prior desensitization therapy are eligible for participation. Primary outcomes will be evaluated one-year post-transplant and defined by a composite endpoint including de novo DSA, biopsy-proven rejection (ACR, AMR), rejection resulting in hemodynamic compromise without biopsy/ histologically-proven rejection, death, or re-transplantation. Planned mechanistic studies test the hypothesis that by inhibiting IL-6R signaling, tocilizumab treatment will alter/ lessen anti-donor Teff immunity, augment Treg number, function, and stability, prevent Tfh differentiation, alter B cell differentiation, diminish DSA production, dampen IRI-induced inflammation and intra-graft fibrogenesis, together resulting in better graft outcomes. Additional investigations will evaluate whether immune alterations detected during tocilizumab treatment persist long-term, testing the hypothesis that early induction of a protective, anti-inflammatory milieu produces long-lasting beneficial effects on the immune system and allograft. To date, randomization is 2/3 complete and the anticipated completion date is early in 2023.
IL-6 inhibition has also been evaluated clinically as a component of desensitization therapy with promising initial results.71,72 In one study, tocilizumab was incorporated into a perioperative desensitization protocol for heart transplant recipients with a positive virtual crossmatch.72 Sensitized recipients (n = 19) received intra-operative tocilizumab in addition to plasma exchange, rituximab, and a 6-month course of IgGAM. Post-transplant outcomes were compared to historical controls consisting of negative crossmatch recipients (n = 98) with no significant difference in rates of ACR or AMR and similar survival at 1 year. It should be noted, however, that this single-arm study lacked a control group of patients treated using the same desensitization regimen without the addition of tocilizumab; randomized, controlled trials are necessary to further evaluate the potential benefit of incorporating IL-6 signaling blockade into a desensitization regimen for heart transplant recipients.
4 |. CONCLUSIONS
Mounting evidence supports the critical role of IL-6 in immune-mediated cardiac allograft injury. Experimental and pre-clinical studies have demonstrated the successful application of IL-6 signaling inhibition for the prevention of acute and chronic cardiac allograft rejection. These promising results provide a compelling basis for current and future clinical trials investigating the use of IL-6 signaling blockade to prevent rejection and ultimately achieve tolerance in human heart transplant recipients.
ACKNOWLEDGMENTS
Research reported in this publication was supported by grants from the National Institutes of Health (NIH): National Institute of Allergy and Infectious Disease (NIAID) P01 AI123086, NIAID Nonhuman Primate Transplantation Tolerance Cooperative Study Group U01 AI131470, and National Heart and Lung Blood Institute (NHLBI) P01 HL158504. CLM was supported by NIAID R25 AI147393, the Nina Starr Braunwald Research Fellowship Award from the Thoracic Surgery Foundation of the Society of Thoracic Surgeons, and a Translational Fellowship Research Grant from the American Society of Transplantation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation Report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37(10):1155–1168. doi: 10.1016/jhealun201807022 [DOI] [PubMed] [Google Scholar]
- 2.Colvin MM, Cook JL, Chang P, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131(18):1608–1639. doi: 10.1161/cir0000000000000093 [DOI] [PubMed] [Google Scholar]
- 3.Smith JD, Banner NR, Hamour IM, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–319. doi: 10.1111/j.1600-6143.2010.03383.x [DOI] [PubMed] [Google Scholar]
- 4.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report–2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1056–1066. doi: 10.1016/jhealun201908004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett. 2011;585(23):3699–3709. doi: 10.1016/jfebslet.201103023 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2010;6(6):843–854. doi: 10.1586/eci.10.70 [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17(9):2381–2389. doi: 10.1111/ajt.14228 [DOI] [PubMed] [Google Scholar]
- 9.Jordan SC, Ammerman N, Choi J, et al. Evaluation of clazakizumab (anti-interleukin-6) in patients with treatment-resistant chronic active antibody-mediated rejection of kidney allografts. Kidney Int Rep. 2022;7(4):720–731. doi: 10.1016/j.ekir.2022.01.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CL, Madsen JC. IL-6 directed therapy in transplantation. Curr Transplant Rep. 2021;8(3):191–204. doi: 10.1007/s40472-021-00331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. doi: 10.1038/nrd.2018.45 [DOI] [PubMed] [Google Scholar]
- 12.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/jimmuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 13.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the Interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation. 2015;99(11):2356–2363. doi: 10.1097/TP.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 14.Lavacca A, Presta R, Gai C, et al. Early effects of first-line treatment with anti-interleukin-6 receptor antibody tocilizumab for chronic active antibody-mediated rejection in kidney transplantation. Clin Transplant. 2020;34(8):e13908. doi: 10.1111/ctr.l3908 [DOI] [PubMed] [Google Scholar]
- 15.Pottebaum AA, Venkatachalam K, Liu C, et al. Efficacy and safety of tocilizumab in the treatment of acute active antibody-mediated rejection in kidney transplant recipients. Transplant Direct. 2020;6(4):e543. doi: 10.1097/TXD.0000000000000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandran S, Leung J, Hu C, Laszik ZG, Tang Q, Vincenti FG. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: a randomized controlled trial. Am J Transplant. 2021;21(7):2543–2554. doi: 10.1111/ajt.16459 [DOI] [PubMed] [Google Scholar]
- 17.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi: 10.1038/s41584-020-0419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doberer K, Duerr M, Halloran PF, et al. A randomized clinical trial of anti-IL-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol. 2020;32:708–722. doi: 10.1681/ASN2020071106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vo AA, Huang E, Ammerman N, et al. Clazakizumab for desensitization in highly sensitized patients awaiting transplantation. Am J Transplant. 2022;22(4):1133–1144. doi: 10.1111/ajt.l6926 [DOI] [PubMed] [Google Scholar]
- 20.Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. 2013;2013:521369. doi: 10.1155/2013/521369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piemonti L, Sordi V, Pellegrini S, et al. Circulating CXCL10 and IL-6 in solid organ donors after brain death predict graft outcomes. Sci Rep. 2021;11(1):6624. doi: 10.1038/s41598-021-86085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater JP, Amirhamzeh MM, Yano OJ, et al. Discriminating between preservation and reperfusion injury in human cardiac allografts using heart weight and left ventricular mass. Circulation. 1995;92(supplement 9):II223–II227. doi: 10.1161/01.cir.92.9.223 [DOI] [PubMed] [Google Scholar]
- 23.Knight RJ, Dikman S, Liu H, Martinelli GP. Cold ischemic injury accelerates the progression to chronic rejection in a rat cardiac allograft model. Transplantation. 1997;64(8):1102–1107. doi: 10.1097/00007890-199710270-00003 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka M, Mokhtari GK, Terry RD, et al. Prolonged cold ischemia in rat cardiac allografts promotes ischemia-reperfusion injury and the development of graft coronary artery disease in a linear fashion. J Heart Lung Transplant. 2005;24(11):1906–1914. doi: 10.1016/jhealun200406007 [DOI] [PubMed] [Google Scholar]
- 25.Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation. 1995;92(7):1866–1875. doi: 10.1161/01.cir.92.7.1866 [DOI] [PubMed] [Google Scholar]
- 26.Gwechenberger M, Mendoza LH, Youker KA, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99(4):546–551. doi: 10.1161/01.cir.99.4.546 [DOI] [PubMed] [Google Scholar]
- 27.Uehara M, Solhjou Z, Banouni N, et al. Ischemia augments alloimmune injury through IL-6-driven CD4(+) alloreactivity. Sci Rep. 2018;8(1):2461. doi: 10.1038/s41598-018-20858-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz J, Thürmel K, Heemann U. Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm. 2010;7:27. doi: 10.1186/1476-9255-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones IKA, Orloff S, Burg JM, et al. Blocking the IL-1 receptor reduces cardiac transplant ischemia and reperfusion injury and mitigates CMV-accelerated chronic rejection. Am J Transplant. 2021;21(1):44–59. doi: 10.1111/ajt.16149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh SSA, Dalzell JR, Berry C, Al-Attar N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail Rev. 2019;24(5):805–820. doi: 10.1007/sl0741-019-09794-l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer A, Kumarasinghe G, Hicks M, et al. Primary graft failure after heart transplantation. J Transplant. 2011;2011:175768. doi: 10.1155/2011/175768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banner NR, Thomas HL, Curnow E, Hussey JC, Rogers CA, Bonser RS. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 2008;86(4):542–547. doi: 10.1097/TP.0b013e31818149b9 [DOI] [PubMed] [Google Scholar]
- 33.Pober JS, Chih S, Kobashigawa J, Madsen JC, Tellides G. Cardiac allograft vasculopathy: current review and future research directions. Cardiovasc Res. 2021;117(13):2624–2638. doi: 10.1093/cvr/cvab259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007;84(6):771–777. doi: 10.1097/01.tp.0000281384.24333.0b [DOI] [PubMed] [Google Scholar]
- 35.Deng MC, Plenz G, Labarrere C, et al. The role of IL6 cytokines in acute cardiac allograft rejection. Transpl Immunol. 2002;9(2–4):115–120. doi: 10.1016/s0966-3274(02)00004-7 [DOI] [PubMed] [Google Scholar]
- 36.Abdallah AN, Billes MA, Attia Y, Doutremepuich C, Cassaigne A, Iron A. Evaluation of plasma levels of tumour necrosis factor alpha and interleukin-6 as rejection markers in a cohort of 142 heart-grafted patients followed by endomyocardial biopsy. Eur Heart J. 1997;18(6):1024–1029. doi: 10.1093/oxfordjournals.eurheartj.a015361 [DOI] [PubMed] [Google Scholar]
- 37.Zhao XM, Frist WH, Yeoh TK, Miller GG. Expression of cytokine genes in human cardiac allografts: correlation of IL-6 and transforming growth factor-beta (TGF-beta) with histological rejection. Clin Exp Immunol. 1993;93(3):448–451. doi: 10.1111/j.l365-2249.1993.tb08199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baan CC, Niesters HG, Balk AH, Mochtar B, Zondervan PE, Weimar W. The intragraft cytokine mRNA pattern reflects the efficacy of steroid antirejection therapy. J Heart Lung Transplant. 1996;15(12):1184–1193. [PubMed] [Google Scholar]
- 39.Perez-Villa F, Benito B, Llancaqueo M, Cuppoletti A, Roig E. Elevated levels of serum interleukin-6 are associated with low grade cellular rejection in patients with heart transplantation. Transplant Proc. 2006;38(9):3012–3015. doi: 10.1016/j.transproceed.2006.08.113 [DOI] [PubMed] [Google Scholar]
- 40.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174(8):4761–4767. doi: 10.4049/jimmunol.174.8.4761 [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 42.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6(4):329–333. doi: 10.1038/nril807 [DOI] [PubMed] [Google Scholar]
- 43.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- 44.von Rossum A, Rey K, Enns W, et al. Graft-derived IL-6 amplifies proliferation and survival of effector T cells that drive alloimmune-mediated vascular rejection. Transplantation. 2016;100(11):2332–2341. doi: 10.1097/TP.0000000000001227 [DOI] [PubMed] [Google Scholar]
- 45.Clerkin KJ, Restaino SW, Zorn E, Vasilescu ER, Marboe CC, Mancini DM. The effect of timing and graft dysfunction on survival and cardiac allograft vasculopathy in antibody-mediated rejection. J Heart Lung Transplant. 2016;35(9):1059–1066. doi: 10.1016/j.healun.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutance G, Ouldamar S, Rouvier P, et al. Late antibody-mediated rejection after heart transplantation: mortality, graft function, and fulminant cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34(8):1050–1057. doi: 10.1016/j.healun.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 47.Hodges AM, Lyster H, McDermott A, et al. Late antibody-mediated rejection after heart transplantation following the development of de novo donor-specific human leukocyte antigen antibody. Transplantation. 2012;93(6):650–656. doi: 10.1097/TP.0b013e318244f7b8 [DOI] [PubMed] [Google Scholar]
- 48.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332(6159):83–85. doi: 10.1038/332083a0 [DOI] [PubMed] [Google Scholar]
- 49.Suematsu S, Matsusaka T, Matsuda T, et al. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A Jan 1. 1992;89(1):232–235. doi: 10.1073/pnas.89.1.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209(7):1241–1253. doi: 10.1084/jem.20120994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol Cell Biol. 2012;90(8):802–811. doi: 10.1038/icb.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 53.Chavele KM, Merry E, Ehrenstein MR. Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J Immunol. 2015;194(6):2482–2485. doi: 10.4049/jimmunol.1401190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen MA, Otten HG, de Weger RA, Huibers MM. Immunological and fibrotic mechanisms in cardiac allograft vasculopathy. Transplantation. 2015;99(12):2467–2475. doi: 10.1097/tp.0000000000000848 [DOI] [PubMed] [Google Scholar]
- 55.Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27(1):21–29. doi: 10.1093/intimm/dxu081 [DOI] [PubMed] [Google Scholar]
- 56.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030 [DOI] [PubMed] [Google Scholar]
- 57.Zhang ZX, Huang X, Jiang J, et al. Natural killer cells play a critical role in cardiac allograft vasculopathy in an interleukin-6--dependent manner. Transplantation. 2014;98(10):1029–1039. doi: 10.1097/TP.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 58.Zhao X, Boenisch O, Yeung M, et al. Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. Am J Transplant. 2012;12(1):90–101. doi: 10.1111/j.1600-6143.2011.03770.x [DOI] [PubMed] [Google Scholar]
- 59.Muckenhuber M, Mengrelis K, Weijler AM, et al. Perioperative IL-6 blockade promotes intra-graft regulation and prevents costimulation-blockade resistant rejection. Am J Transplant. 2022;22(supplement 3):543–544. [Google Scholar]
- 60.Booth AJ, Grabauskiene S, Wood SC, Lu G, Burrell BE, Bishop DK. IL-6 promotes cardiac graft rejection mediated by CD4+ cells. J Immunol. 2011;187(11):5764–5771. doi: 10.4049/jimmunol.ll00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma M, Sun Q, Li X, et al. Blockade of IL-6/IL-6R signaling attenuates acute antibody-mediated rejection in a mouse cardiac transplantation model. Front Immunol. 2021;12:778359. doi: 10.3389/fimmu.2021.778359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant. 2009;9(8):1773–1783. doi: 10.1111/j.1600-6143.2009.02706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solhjou Z, Uehara M, Bahmani B, et al. Novel application of localized nanodelivery of anti-interleukin-6 protects organ transplant from ischemia-reperfusion injuries. Am J Transplant. 2017;17(9):2326–2337. doi: 10.1111/ajt.14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tonsho M, Benichou G, Boskovic S, et al. Successful tolerance induction of cardiac allografts in nonhuman primates through donor kidney co-transplantation. Am J Transplant. 2013;13(supplement 5):48. [Google Scholar]
- 65.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. [PubMed] [Google Scholar]
- 66.Yang C, Ge J, Rosales I, et al. Kidney-induced systemic tolerance of heart allografts in mice. JCI Insight. 2020;5(18):e139331. doi: 10.1172/jci.insight.l39331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aoyama A, Klarin D, Yamada Y, et al. Low-dose IL-2 for In vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. Am J Transplant. 2012;12(9):2532–2537. doi: 10.1111/j.l600-6143.2012.04133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahrens KJ, Miller CL, O JM, et al. Effects of IL-2 and/or anti-IL6R therapy on long-term cardiac allograft survival in non-human primates. J Heart Lung Transplant. 2021;40(supplement 4):S137. doi: 10.1016/j.healun.2021.01.426 [DOI] [Google Scholar]
- 69.Ahrens KJ, O JM, Sommer W, et al. IL-6 receptor blockade but not IL-2 treatment contributes to long-term NHP cardiac allograft survival in transient mixed chimeras. Am J Transplant. 2020;20(supplement 3):S70–S71. [Google Scholar]
- 70.Miller CL, Ahrens KJ, O JM, et al. Successful use of anti-IL-6R therapy to achieve cardiac allograft tolerance in non-human primates. J Heart Lung Transplant. 2022;41(supplement 4):S148. doi: 10.1016/j.healun202201349 [DOI] [Google Scholar]
- 71.Deen J, Patel J, Kittleson M, et al. Efficacy of tocilizumab for refractory sensitized patients awaiting heart transplantation. J Heart Lung Transplant. 2022;41(supplement 4):S339. doi: 10.1016/j.healun.2022.01.1402 [DOI] [Google Scholar]
- 72.Sommer W, Avsar M, Aburahma K, et al. Heart transplantation across preformed donor-specific antibody barriers using a perioperative desensitization protocol. Am J Transplant. 2022;22(8):2064–2076. doi: 10.1111/ajt.l7060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


