Abstract
DNA origami has emerged as a powerful method to generate DNA nanostructures with dynamic properties and nanoscale control. These nanostructures enable complex biophysical studies and the fabrication of next-generation therapeutic devices. For these applications, DNA origami typically needs to be functionalized with bioactive ligands and biomacromolecular cargos. Here, we review methods developed to functionalize, purify, and characterize DNA origami nanostructures. We identify remaining challenges, such as limitations in functionalization efficiency and characterization. We then discuss where researchers can contribute to further advance the fabrication of functionalized DNA origami.
ToC blurb
DNA origami nanostructures are useful constructs for biophysical and therapeutic studies. In this Review, we discuss how these nanostructures are functionalized with bioactive conjugates, purified, and characterized, and we compare the advantages and limitations of these methods in the context of different applications.
Introduction
The DNA origami method enables high-yield fabrication of DNA nanostructures at the 1–100 nm length scale1,2. In this method, a long single-strand DNA (ssDNA) ‘scaffold’ is folded into a desired shape using short oligonucleotide ‘staple’ strands via Watson-Crick base pairing. The DNA origami method can now generate 2D and 3D bricklike geometries3–5, intricate wireframe assemblies6,7, and higher-order superstructures8–10. For challenging sequence designs, top-down design algorithms have enabled rapid prototyping of nanostructures and democratized this technique to a broader range of scientists and engineers6,7,11–15. Fabrication advances in custom scaffolds16–21, biological22 and enzymatic23,24 staple synthesis, and scaled-up thermal annealing25 now allow for large-scale production of DNA origami. With several bottlenecks in DNA origami nanostructure design and fabrication overcome, DNA origami is widely applied to probe biological systems and has potential as a translational biomaterial26–28.
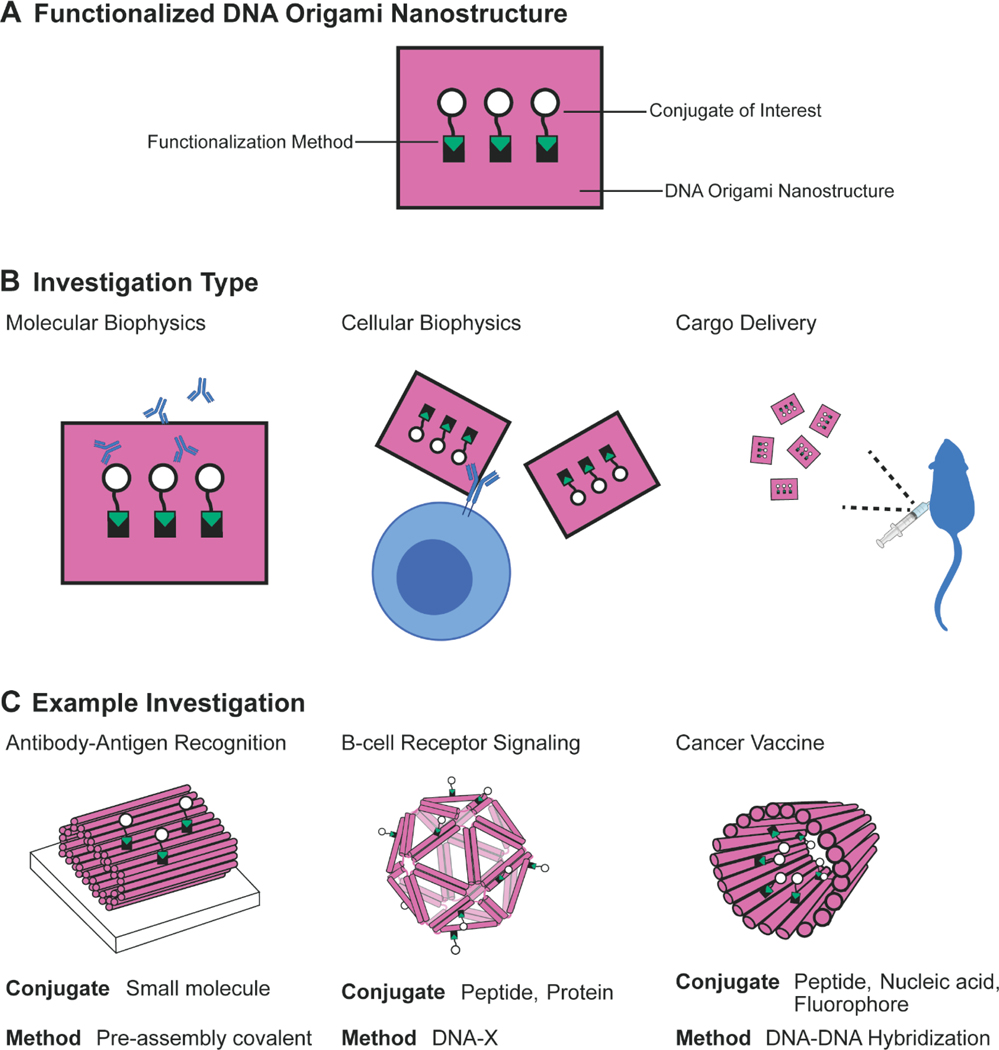
DNA origami is particularly useful when applied to interrogate and interface with biological systems owing to its ability to position bioactive moieties at the nanometer length scale29–31. For example, functionalized DNA origami nanostructures (Figure 1a) have been used to determine the nanoscale properties governing immune receptor activation32–36 and enzyme activities37–39. Enzyme cascade systems can be enhanced by programming molecular motion into nanoscale enzyme arrays40. DNA origami nanostructures functionalized with small molecule antigens at different nanoscale spacings are able to determine the spatial tolerances of antibody-antigen interactions41, and functionalized icosahedral nanostructures have revealed spatial rules of B cell receptor activation32.
Figure 1. Functionalized DNA origami nanostructures interact with biological systems.
a | Functionalized DNA origami nanostructures have conjugates of interest attached to DNA origami nanostructures using specific functionalization methods. b | These functionalized nanostructures can interact with biological systems at different length scales to enable various biological experiments. c | Examples of functionalized DNA origami nanostructures utilized in each investigation type: determining the spatial tolerance of antibody-antigen recognition41; determining the spacing rules of B-cell receptor signaling32; and investigating a cancer vaccine in vivo45. Panel C (Left) adapted with permission from ref.41, Springer Nature Limited. Panel C (Center) adapted with permission from ref.32, Springer Nature Limited. Panel C (Right) adapted with permission from ref.45, Springer Nature Limited.
DNA origami also has the ability to compute, performing Boolean logic in therapeutic applications to deliver small molecules, nucleic acids, and therapeutic proteins in vitro42 and in vivo43,44. For example, in animal models, a modular DNA origami nanotube delivered therapeutic proteins to tumors43, antigenic peptides and adjuvants to dendritic cells45, and siRNA and chemotherapeutic drugs to tumors44. These delivery studies highlight that a single DNA origami nanostructure can be utilized in widely different applications, depending on what it is functionalized with. This modularity positions DNA origami as an emerging disease-agnostic biomaterial for a broad range of therapeutic delivery and vaccine applications.
All of the aforementioned examples can broadly be classified as molecular biophysics studies, cellular biophysics studies, or cargo delivery studies (Figure 1b). For each of these applications, the DNA origami nanostructure needs to be functionalized with a wide range of bioactive moieties, from small molecules to large proteins. Each type of study also requires different functionalization techniques, fabrication scales, stabilities, and costs, leading to a specific set of suitable methods (Figure 1c).
In this Review, we describe how DNA origami is functionalized, purified, and characterized, and we discuss advantages and limitations of each approach in the context of different applications. We identify where innovation in this space is needed to further advance the field, as well as areas of investigation that would help facilitate the translation of DNA origami towards clinical applications.
Functionalizing DNA origami
DNA origami nanostructures are fabricated, as reviewed elsewhere46, by designing staple sequences, manually or algorithmically, for a given scaffold; producing the scaffold and staple strands; combining the scaffold and staple strands in buffer and annealing the mixture; and purifying and characterizing the resulting DNA nanostructures.
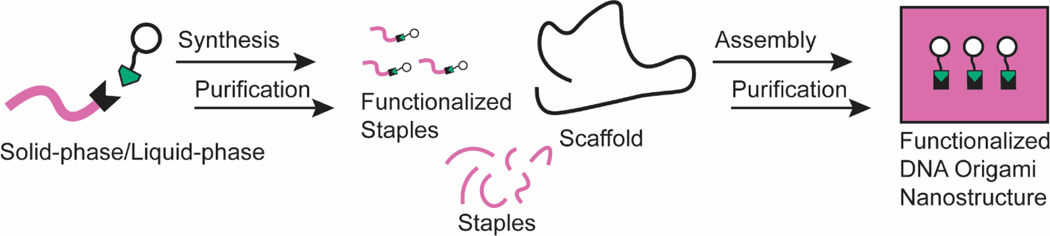
There are two typical workflows to functionalize DNA origami. Individual components of the DNA nanostructure can be functionalized before the nanostructure is self-assembled (Figure 2), or the entire nanostructure can be functionalized following its self-assembly (Figure 3a). For the scope of this Review, we define ‘functionalization’ as the attachment of distinct chemical moieties onto the DNA nanostructure.
Figure 2. A typical workflow for pre-assembly functionalization of DNA origami.
Staples are modified before assembly with the desired conjugate, either through solid-phase or liquid-phase chemistry. Modified staples are then assembled with the scaffold and other staples to yield a functionalized DNA origami nanostructure.
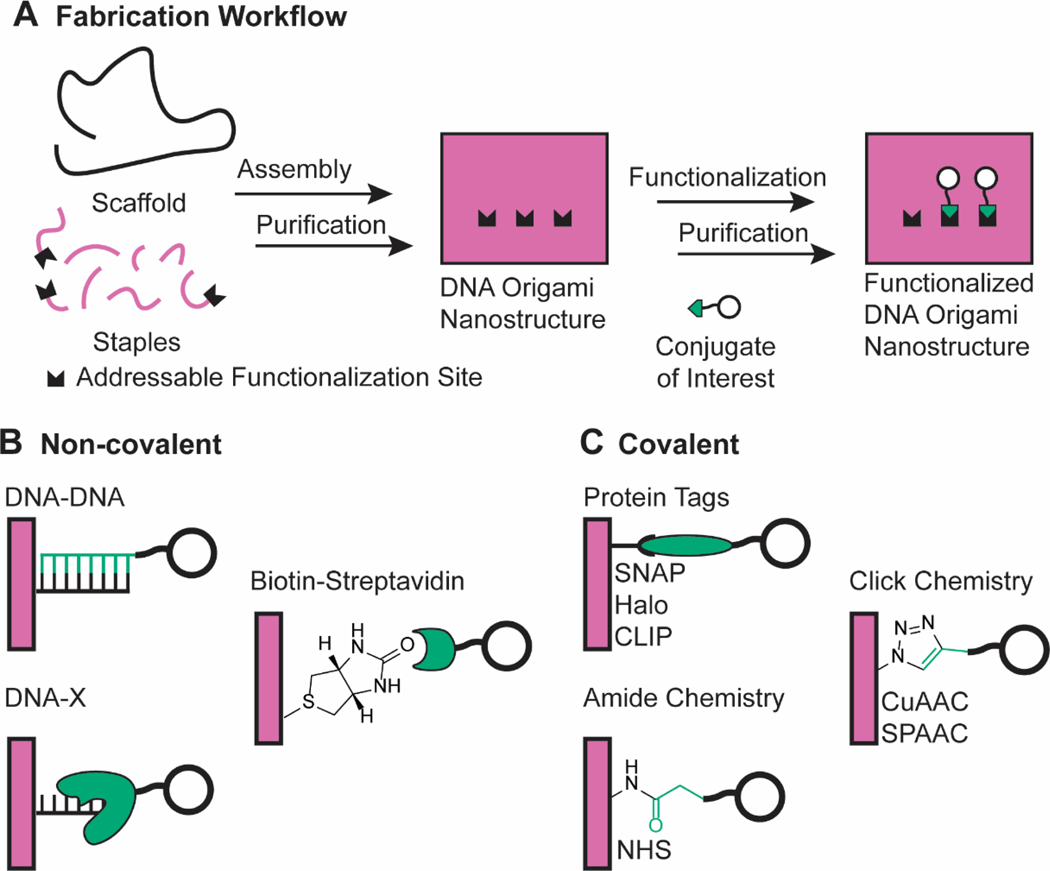
Figure 3. A typical workflow for post-assembly functionalization of DNA origami.
a | Staples with addressable functionalization sites are assembled with the scaffold and other staples into DNA nanostructures. The conjugate of interest is then incorporated into the nanostructure through non-covalent or covalent methods. b | Non-covalent strategies attach conjugates onto DNA nanostructures through non-covalent molecular interactions. c | Covalent strategies rely on the formation of a covalent bond between the nanostructure and the conjugate.
Pre-assembly functionalization
In pre-assembly functionalization, conjugates of interest are covalently bound to staple strands that are then integrated into the DNA nanostructure during self-assembly (Figure 2). One advantage of this strategy is that the valency of conjugates on the designed DNA nanostructure is known and coverage is quantitative per conjugation site, given the purity of the staple conjugates and the quantitative integration of staples into the nanostructure. In addition, the covalent attachments between the conjugates and DNA nanostructure potentially benefit the stability of the assembly. Pre-assembly functionalization can be performed in either the solid or liquid phase, providing far greater control over reaction conditions (such as concentration or co-solvents) than post-assembly functionalization methods.
Pre-assembly techniques have been used to conjugate fluorophores35,36,47–86, lipids33,50,56,57,60,66,80,87–89, small molecules35,36,41,63,85,90–100, aptamers84,101–104, peptides56,105,106, and polymers107 to DNA origami. These techniques are especially common for incorporating fluorophores, as they are attached easily to staples during solid-phase synthesis of the oligonucleotides using phosphoramidite chemistry. Such constructs have been used as a biosensor72, to investigate cellular uptake48, and to silence genes in plants77. Lipids attached to DNA origami displaying stimulatory ligands with specific spatial arrangements have been used to investigate intracellular signaling of T cells33. By incorporating the lipids into the DNA nanostructures prior to assembly, the nanostructures could later be anchored to a lipid bilayer and used to determine the minimal signaling unit promoting efficient T cell activation. Aptamers can also be integrated into the scaffold of the nanostructure through scaffold sequence design, for example for targeted cancer therapy108. Scaffold functionalization offers the potential for high conjugate density on nanostructures; this is an underexplored area that we believe has potential to expand the DNA origami functionalization toolkit.
Although useful in numerous applications, pre-assembly functionalization has two major disadvantages. First, it is incompatible with conjugates that are sensitive to the high temperatures and high salt concentrations needed for DNA nanostructure self-assembly, such as proteins that can denature and aggregate. The second disadvantage is that functionalized staples need to be individually synthesized, purified, and characterized. This bottleneck can limit the throughput and thus ability to prototype nanostructures through the generation of libraries, a common technique to explore promising therapeutic delivery nanotechnologies such as lipid nanoparticles109,110. Now that other workflow bottlenecks in DNA origami structural design and fabrication have been overcome, functionalization workflows that afford the ability to generate material libraries are particularly important to enable the rapid exploration of DNA origami chemical design space.
Post-assembly functionalization
As an alternative, conjugates of interest can be attached to the nanostructure after it is self-assembled, either through non-covalent or covalent approaches. Non-covalent approaches, such as the popular DNA-DNA hybridization method, were initially developed for their ease of implementation and today are the most commonly reported methods to functionalize DNA origami (Figure 3b). They do suffer from limitations in select applications, however, and covalent approaches can help fill in these gaps.
Non-covalent functionalization
DNA-DNA hybridization is the most common non-covalent approach. This method relies on ssDNA overhangs, typically 15–25 nucleotides long, which are extended from the DNA nanostructure. The desired conjugate of interest is chemically attached to another ssDNA handle that has a complementary sequence to the overhangs. After the nanostructure is assembled, it is incubated with the conjugate-DNA molecule to produce the functionalized nanostructure. This technique can attach proteins34,37–40,42,43,54,55,59,61,73,75,78,79,89,92,104,107,111–152, fluorophores43,79,100,114,115,122,130,131,136,142,143,151,153–169, aptamers81,170–178, RNA123,179–181, small interfering RNA44,77,160,182, short hairpin RNA67, antisense oligonucleotides171, peptides32,44,45,152,156,183-187, lipids62,82,89,141,148,157,165,167–169,188–194, metals42,59,75,103,122,124,126,150,151,162,182,195–201, quantum dots202, genomic DNA203, polymers107, and branched oligonucleotides204 to DNA nanostructures. The sequence-programmable, heterovalent nature of the method made it possible to orthogonally attach antigenic peptides, adjuvanting nucleic acids, and fluorophores to DNA nanostructures to fabricate an environmentally-responsive cancer vaccine45. DNA-DNA hybridization suffers from loss of nanoscale addressability, however, as hybridization sites can span several nanometers in length, and risks DNA-DNA dissociation and loss of the conjugate. These design limitations are in competition because increasing hybridization length to increase thermodynamic stability correspondingly decreases nanoscale spatial resolution. Additionally, exposed duplexes generate additional susceptibility to nuclease degradation.
A technique similar to DNA-DNA hybridization is DNA-X hybridization, in which specific DNA-recognizing moieties, such as peptide nucleic acids (PNAs) and DNA-binding proteins, are used as handles to attach to ssDNA overhangs. DNA-PNA hybridization attached a clinically relevant HIV antigen to virus-like wireframe DNA origami particles, which were then used to study antigen-B-cell receptor interactions at the nanoscale32. DNA-binding protein interactions also enable site-specific protein positioning. In the initial report of this strategy, nanostructures incorporated with zinc-finger-binding DNA motifs could then conjugate with zinc-finger proteins of interest205. This technique has been extended to other DNA-protein binding systems206, and was utilized for structural biology65,207,208, such as for single particle cryogenic electron microscopy of the DNA-binding proteins themselves209. DNA-X hybridizations have stronger interaction strengths than DNA-DNA hybridization, leading to higher fidelity bonds and more efficient fabrication protocols. However, DNA-X systems are often more expensive and require more expertise to implement.
Biotin-streptavidin functionalization relies on the high affinity non-covalent interaction between the streptavidin protein and the biotin small molecule. This method is easily accessible due to the ease of installing biotin onto staples, and the ease of engineering streptavidin systems for many purposes. It is often used to functionalize nanostructures with proteins33,47,57,58,89,96,105,119,185,210–219, but can also attach peptide220 and metal221 conjugates. However, streptavidin-based functionalization requires modifying the streptavidin protein itself with a conjugate in a site-selective, stoichiometric manner, which can be challenging for non-protein conjugates. Additionally, the large size of streptavidin itself limits nanoscale addressability. Finally, when used in cargo delivery investigations, streptavidin can also cause undesired immunogenicity222,223.
The choice of functionalization strategy can influence the outcome of mechanistic studies in which DNA origami is used. In a comparative analysis of DNA-DNA hybridization, DNA-X hybridizations, and streptavidin-biotin systems, each method was evaluated for its efficiency in attaching a TCR-activating ligand to DNA origami, and for the functionalized construct to activate T cells89. Although the functionalization efficiencies were similar between the methods, importantly, activation levels differed significantly. Specifically, the DNA-DNA hybridization method that is commonly used to functionalize nanostructures with proteins decreased the functionality of the TCR-activating ligand once attached, attributed to the negatively charged DNA hybridization linker interfering with TCR binding. In contrast, decreases in activity were not observed with the biotin-streptavidin and the DNA-PNA hybridization systems.
There are several other methods to non-covalently functionalize DNA origami. Metal-nitrilotriacetic acid-His coordination bonds can pattern proteins onto DNA nanostructures224,225. Intercalation between DNA duplexes is a common and simple way to load small molecules in nanostructures, particularly chemotherapeutic drugs such as doxorubicin and daunorubicin44,67,83,163,164,174,182,200,203,221,226–236. However, researchers have identified doxorubicin aggregation mechanisms and spectral dynamics that complicate this functionalization strategy236. Complexation methods utilizing physical interactions between the negatively charged phosphate backbones of DNA duplexes and the conjugate allow for highly dense functionalization with proteins49,237–242, fluorophores243, polymers100,151,168,202,244–249, peptides86,250, lipids251, and small molecules252. However, complexation and intercalation strategies typically lack nanoscale spatial control of conjugate positioning and stoichiometric control of conjugate copy number per nanostructure, which are two of the key advantages of DNA origami nanotechnology.
Covalent functionalization
Various bioconjugation and general covalent chemistries have been adopted to covalently functionalize DNA origami nanostructures (Figure 3c). Protein tags are specific peptide and protein motifs that rapidly and efficiently form covalent bonds with a small molecule253. SNAP217,253–257, HALO166,217,253,255,256,258–260, and CLIP255,260 tags, as well as more efficient, rationally engineered tags261, have conjugated proteins to DNA origami. Combining these protein tags with DNA-protein binding systems can create orthogonal covalent chemistries with enhanced kinetics and functionalization efficiencies255,257,260. Protein tag functionalization strategies employ mild conditions beneficial to both the nanostructure and the conjugate. However, it requires additional protein engineering of the conjugate and is limited in conjugate scope. Additionally, these tags could potentially lead to undesired immunogenicity in cargo delivery investigations, as noted for streptavidin systems.
N-Hydroxysuccinimide (NHS) esters react with a primary amine to form an amide bond. They are common bioconjugation reagents262 owing to the prevalence of primary amines in biomacromolecules, such as the lysine residue. Amine groups are easily integrated into staple strands, and a wide range of NHS-functionalized molecules are commercially available. This reaction chemistry is more general than protein tags and has been used to conjugate proteins263, metals112, and small molecules264. Still, this method suffers from poor functionalization efficiency in aqueous environments and has thus rarely been implemented in the DNA origami literature since its initial demonstration.
Click chemistries265 are well-suited to address the above limitation owing to their superior reaction thermodynamics and kinetics in aqueous environments. Click moieties, such as azides and alkynes, are readily incorporated into staple strands and desired conjugates. Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) chemistry was first demonstrated on a DNA origami sheet to attach a small molecule264, and has since been extended to attach proteins122 and to stabilize nanostructures through staple-staple conjugations266. Strain-promoted azide-alkyne cycloaddition (SPAAC) has slower reaction kinetics than CuAAC, but benefits from not requiring the copper catalyst, which is harmful to biological systems267 and is complicated to implement in DNA-based systems268. SPAAC has been used to polymerize monomeric origami units269, densely decorate nanostructures with fluorophores270, and functionalize wireframe DNA origami nanostructures with proteins, peptides, polymers, carbohydrates, and fluorophores271.
Compared to non-covalent methods, covalent methods address two key fabrication challenges. First, because covalent bonds are more stable than non-covalent bonds, covalently functionalized DNA nanostructures are less likely to lose conjugates of interest. Conjugation stability and biodegradability are likely to become critical design parameters as these nanostructures are increasingly utilized for therapeutic payload delivery in vivo. Second, covalent strategies may enable conjugates to be spaced more precisely on nanostructures; high spatial resolution is often a critical design parameter, in particular in molecular and cellular biophysics investigations. A disadvantage of covalent methods is that they require a large number of staples containing functionalization sites, which may either be expensive to purchase or require in-house equipment and technical expertise to prepare. Additionally, the kinetics of some covalent reactions, such as SPAAC272, are slower than those of non-covalent reactions273,274, making it more challenging to achieve high functionalization efficiency.
Considerations and analysis
Depending on the investigation type, each functionalization method has different advantages and disadvantages (Table 1). When choosing which method to use for a given investigation, the following considerations are important: the compatibility of the workflows with the conjugate of interest (for example, can the conjugate survive self-assembly conditions?); whether achieving quantitative functionalization efficiency is essential; whether the long-term and/or biological integrity of the conjugate-nanostructure bond are important; and the amount of the functionalized DNA origami nanostructure to be fabricated. From a manufacturability perspective, short reaction times and low required equivalents of conjugate may benefit DNA origami as a translational biomaterial.
Table 1.
DNA origami functionalization strategies.
| Functionalization technique | Advantages | Disadvantages | Conjugate scopea | |
|---|---|---|---|---|
|
| ||||
| Pre-assembly | ||||
|
| ||||
| Covalent | Simple synthesis Simple characterization High functionalization efficiency Broad conjugate scope Simple heterovalent display |
Not implementable with protein conjugates Inefficient workflow if many staples need to be functionalized Inefficient workflow if many different conjugates need to be functionalized |
Fluorophores Lipids Small molecules Aptamers Peptides Polymers |
|
|
| ||||
| Post-assembly | ||||
|
| ||||
| Non-covalent | DNA-DNA | Broadest conjugate scope Most method development |
Possible site of nuclease degradation Less spatial control |
Proteins Fluorophores |
|
| ||||
| Simple heterovalent display | Lipids Small molecules Aptamers Peptides Polymers siRNA ASO Metals Misc. oligonucleotides |
|||
|
|
||||
| DNA-X | Stronger non-covalent bonding than DNA-DNA High functionalization efficiency |
Minimal conjugate scope Expensive, or requires technical expertise |
Proteins | |
|
|
||||
| Biotin-Streptavidin | Extremely strong non-covalent bond | Minimal conjugate scope Large tag incorporated onto conjugate of interest |
Proteins Peptides |
|
|
|
||||
| Metal-NTA | Orthogonal to other methods | Minimal conjugate scope Minimal development |
Proteins | |
|
|
||||
| Intercalation | Simple synthesis Dense functionalization |
Lack of stoichiometric control Lack of spatial control Minimal conjugate scope |
Small molecules | |
|
|
||||
| Complexation | Simple synthesis Dense functionalization Broad conjugate scope |
Lack of stoichiometric control Lack of spatial control Minimal conjugate scope |
Proteins Fluorophores Lipids Small molecules Peptides Polymers |
|
|
| ||||
| Covalent | Protein Tags | High functionalization efficiency Heterovalent display |
Minimal conjugate scope Requires expertise in protein engineering Large tag incorporated onto conjugate of interest |
Proteins |
|
|
||||
| NHS-ester | Simple synthesis | Low functionalization efficiency Minimal development Difficult to implement heterovalent display |
Proteins Small molecules Metals |
|
|
|
||||
| Click chemistry | High functionalization efficiency | Expensive, or requires technical expertise | Proteins Fluorophores |
|
|
|
||||
| Broad conjugate scope | Difficult to implement heterovalent display |
Small molecules Peptides Polymers Carbohydrates |
||
Conjugate scope is not the theoretical conjugate scope, but rather the scope that has been demonstrated in the literature. ASO: antisense oligonucleotide. siRNA: small interfering RNA.
Nanoparticles that are heterovalent—that is, nanoparticles displaying more than one type of molecule on their surface—are useful in many areas, including vaccinology275,276. Pre-assembly functionalization methods, and the post-assembly non-covalent DNA-DNA and DNA-X hybridization methods, can easily produce nanostructures with heterovalency. In the case of pre-assembly methods, each staple can be functionalized with a different conjugate. For the DNA-DNA and DNA-X hybridization methods, the sequence-programmable nature of DNA allows multiple different conjugates to be attached to specific parts of the nanostructure based on unique DNA overhang sequences. Introducing heterovalency through covalent methods is more challenging, as orthogonal, efficient reactive groups have to be installed onto both the staples and conjugates. Heterovalency has been demonstrated with protein tag functionalizations253,255, but not with other covalent chemistry systems, or for conjugates other than proteins.
Purifying functionalized DNA origami
Once DNA origami nanostructures are functionalized, they typically need to be purified (Figure 4). This purification step is required even when conjugates are attached with high functionalization efficiency, because conjugates are added in excess. With pre-assembly functionalization strategies, excess staples, excess functionalized staples, non-folded scaffolds, and misfolded nanostructures are purified away from functionalized nanostructures. When post-assembly strategies are implemented, excess conjugates are purified away from functionalized nanostructures. In both cases, methods typically used to purify non-functionalized DNA origami nanostructures, as reviewed elsewhere46,277, are employed.
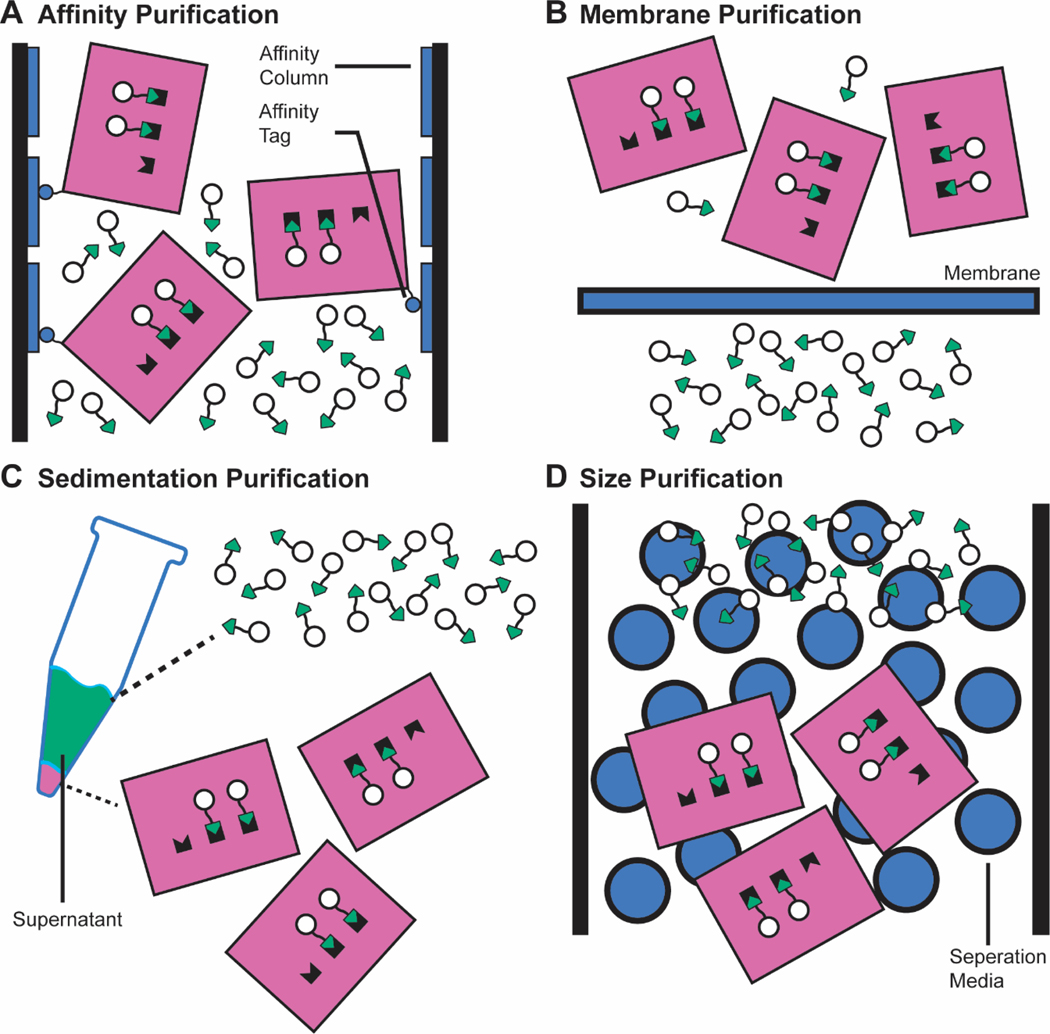
Figure 4. Methods to purify functionalized DNA origami nanostructures.
Purification methods aim to separate functionalized nanostructures from other components in the reaction mixture. In pre-assembly workflows, these methods purify away unfolded scaffolds, excess staples, excess functionalized staples, and misfolded nanostructures. In post-assembly workflows, excess conjugates, and possibly catalysts or side products, are purified away from the functionalized nanostructure. a | Affinity purification methods rely on the affinity between a specific tag on nanostructures and an external material, either column walls or magnets. b | Membrane purification methods utilize membranes that separate reaction mixtures components by a size cut-off. c | Sedimentation purification methods utilize the difference in sedimentation rates between components in the reaction mixture during centrifugation, either in buffer or glycerol solutions. d | Size purification methods rely on different retention times of reaction mixture components in different media, such as agarose gels and SEC beads.
Affinity purification
Affinity purification techniques rely on attractive interactions between the functionalized DNA origami, which has been labeled with affinity tags, and the purification equipment to separate the nanostructure from the untagged conjugates (Figure 4a). In its first implementation, nanostructures with His-tag-modified staple strands were purified on a cobalt-based metal affinity resin, owing to the affinity between the His-tag and metal ions105. His-tags are commonly used for the purification of proteins themselves, and therefore may not be suitable for purifying functionalized DNA nanostructures when the conjugate of interest is a protein.
Magnetic beads can also provide the affinity for this type of purification. In the most common version of this approach, biotinylated staples are first incorporated into the nanostructure. After functionalization, the nanostructures are incubated with, and become affixed to, streptavidin-coated magnetic beads. Nanostructures can then be pulled from the rest of the solution containing excess conjugates with a magnet73,133,146,147,219,261. Finally, the functionalized nanostructures are released from the magnetic beads through competitive binding that displaces the biotin-streptavidin bond. This strategy has also been performed using DNA hybridization between a DNA origami gene logic chip tagged with ssDNA overhangs and complementary ssDNA-labelled magnetic beads254. This technique was extended further to perform solid-phase synthesis of DNA origami-protein nanostructures; the nanostructure is first attached to magnetic beads through biotin-streptavidin interactions, then functionalized with protein, and then purified from excess protein before being removed from the magnetic beads258. Although magnetic purification has mainly been used to separate protein conjugates from functionalized DNA origami, they are implementable with other conjugate classes such as fluorophores131.
Membrane purification
Membrane purification techniques utilize a membrane with a size-based cut-off to separate large nanostructures from small conjugates. Centrifugal filtration, which couples such a membrane with centrifugation to speed up the process (Figure 4b), is by far the most common approach to purify functionalized DNA origami. It is suitable for nanostructures functionalized with proteins32,33,42,43,89,96,104,111,112,128,131,148,152,206,213,215,216,224,271, peptides32,44,45,152,187,220,271, metals42,112,182,198,200, fluorophores43,82–85,131,158,165,167,168,243,270,271, lipids62,82,88,89,148,165,167,168,189, small molecules83,85,182,228,234,271, polymers168,244,271, and nucleic acids44,45,84,108,170,171,175,177,178,182,278. When purifying protein-functionalized nanostructures, using protein-61,131 or surfactant-coated32,131,271 membranes can increase the recovery yield. Centrifugal filtration is viable for preclinical in vivo investigations because the filters are commercially available at large volumes43,45. However, as the conjugates get larger, more purification rounds are required due to less efficient separations, possibly leading to more impurities or lower purification recovery yields. Centrifugation-based methods also have limitations when approaching clinical or industrial scales.
Dialysis is a membrane-based method that separates excess conjugates into a reservoir buffer through diffusion. Because it limits local concentration gradients and centrifugal forces, dialysis is milder than centrifugal filtration and is feasible at larger scales, although the speed of purification is substantially slower. It has been used to separate nanostructures functionalized with proteins253 and lipids188–190,192.
Sedimentation purification
Centrifugation can separate functionalized nanostructures from conjugates by taking advantage of their different rates of sedimentation (Figure 4c). The most basic method, often employed to separate small molecule drugs like doxorubicin from the functionalized nanostructures164,174,227,233,236, is to simply centrifuge the sample in aqueous buffer, discarding the conjugates remaining in the supernatant137. However, centrifugation suffers from low molecular weight resolution, leading to limited applicability.
Rate-zonal centrifugation has a broader range of applicability. It was initially developed as a scalable, non-laborious method to purify DNA nanostructures after self-assembly with high separation resolution279. In a typical implementation, reaction mixtures containing functionalized nanostructures and excess conjugates are incubated in glycerol gradient solutions and then centrifuged, resulting in separated fractions of nanostructures and conjugates. This separation occurs because the different components sediment through the gradient at different rates during centrifugation. For functionalized DNA origami nanostructures, this method can purify away protein117,123,126,131,134,140,141,143,150, lipid141,189,191, nucleic acid123,126, metal126,150, and fluorophore143 conjugates, showcasing its broader scope compared to centrifugation. A drawback is that it struggles to obtain high concentrations of pure functionalized DNA origami samples. High concentrations of nanostructures can be necessary, for example during structural characterization with cryo-EM, and for administration in animal models where there are upper limits of injection volumes.
PEG precipitation can overcome this limitation by giving high recovery yields of the purified nanostructure280. Large molecular weight nanostructures are separated from lower molecular weight conjugates due to excluded volume effects from the addition of PEG molecules. Nanostructures are precipitated from solution due to PEG crowding, and then resuspended into a buffer of choice at a desired concentration. It can purify DNA origami nanostructures functionalized with proteins59,65,79,122,127,130,131,149,206, fluorophores35,36,48,56,79,81,122,130,131,153,154, metals59,122, peptides56, aptamers81,173,176, small molecules35,36,97,98, and lipids56. However, this method leaves residual PEG molecules in samples46, potentially disrupting some investigations, for example due to the possible immunogenicity of PEG.
Size purification
Size purification techniques, like membrane purification, rely on a material’s ability to separate molecules based on size (Figure 4d). Size-exclusion chromatography (SEC) uses a column packed with porous beads to separate smaller and larger molecules from each other due to different size-dependent retention times within the pores. SEC can purify DNA origami nanostructures functionalized with protein34,38,49,131,255,257, fluorophore80,131,154,163, and lipid80 moieties. SEC is able to purify large amounts of material in principle, and SEC-based purification technologies have scaled to industry in multiple biotechnological contexts281,282.
Gel filtration is similar to SEC, except it uses centrifugation rather than pump- or gravity-driven flow to send sample through the column. Gel filtration has purified DNA origami nanostructures functionalized with proteins131,132,166,205,218,260, fluorophores71,72,131,161,166, peptides186, and polymers249. An advantage of gel filtration over SEC is that it requires no specialized chromatography instrumentation; however, purification scales are limited by the volume capacities of the gel column and centrifuge.
Gel purification relies on electrophoresis to sort materials through a gel, commonly agarose-based. As an applied electric field drives the sample through the gel, materials of different size and charge migrate differently in the media, forming bands of pure substances that can be collected by excising the band, dissolving the gel, and exchanging the buffer to yield a pure sample. Gel purification has higher resolution than SEC, and has been utilized to purify functionalized nanostructures with polymers107, fluorophores50,52,67,78,131,162, lipids50,199, metals151,162,197,199, small molecules155,203, nucleic acids67,179,203, and proteins78,118,131,144,151,263, demonstrating its broad utility. One limitation of this approach is scalability. It is typically challenging to obtain sufficient quantities of functionalized nanostructure for preclinical in vivo investigations.
Free-flow electrophoresis purification uses similar principles for separation as gel purification, but occurs in a matrix-free (buffer only) environment. It was introduced to overcome limitations of other methods, namely speed and chemical/mechanical stress, in purifying protein-functionalized DNA origami nanostructures217. With this method, purified functionalized DNA origami can be obtained rapidly (~10–15 minutes) under mild physicochemical conditions with moderate recovery yields (50–75%), but it is unknown how it would perform with nanostructures functionalized with non-protein conjugates.
Considerations and analysis
Considerations of conjugate type, fabrication scale, equipment requirements, purity requirements, and end-use concentration requirements are important to identify an appropriate purification method. When deciding, one might consider whether purification after functionalization is necessary, whether impurities introduced during purification (e.g., PEG purification) are acceptable, what scale of functionalized DNA origami nanostructure needs to be purified, and what the end-use concentration requirement of functionalized nanostructure is.
A comparative study examined the ability of seven purification methods—centrifugal filtration, gel filtration, rate-zonal centrifugation, PEG precipitation, gel purification, magnetic purification, and SEC—to purify fluorophore- and protein-functionalized nanostructures131. Although for most methods the recovery yield depended on the conjugate, the recovery yield for magnetic bead capture was independent of the conjugate’s properties, suggesting its potential as a universal purification method. Methods that tend to concentrate nanostructure samples, such as PEG precipitation, led to aggregation of the nanostructures.
Characterizing functionalized DNA origami
One important characteristic of DNA origami that distinguishes it from other soft-material nanotechnologies is its ability to control the stoichiometry and nanoscale organization of conjugates. However, in order to realize this control, characterization methods need to distinguish between fully functionalized and partially functionalized nanostructures. Functionalization efficiency that is lower than desired may influence the outcome of mechanistic studies, as well as the efficacy of therapeutics. Additionally, the absence of adequate characterization may lead to the misinterpretation of investigative results.
Generally, there is a lack of suitable characterization methods to analyze the functionalization efficiency of DNA origami283, in part because of nanostructure concentration ranges that result from fabrication. Typical reaction characterization techniques from other soft-matter nanotechnology or organic chemistry contexts, such as infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance spectroscopy, are not suitable for characterizing functionalized DNA origami. Lipid nanoparticles have had similar characterization challenges during their developmental arc284.
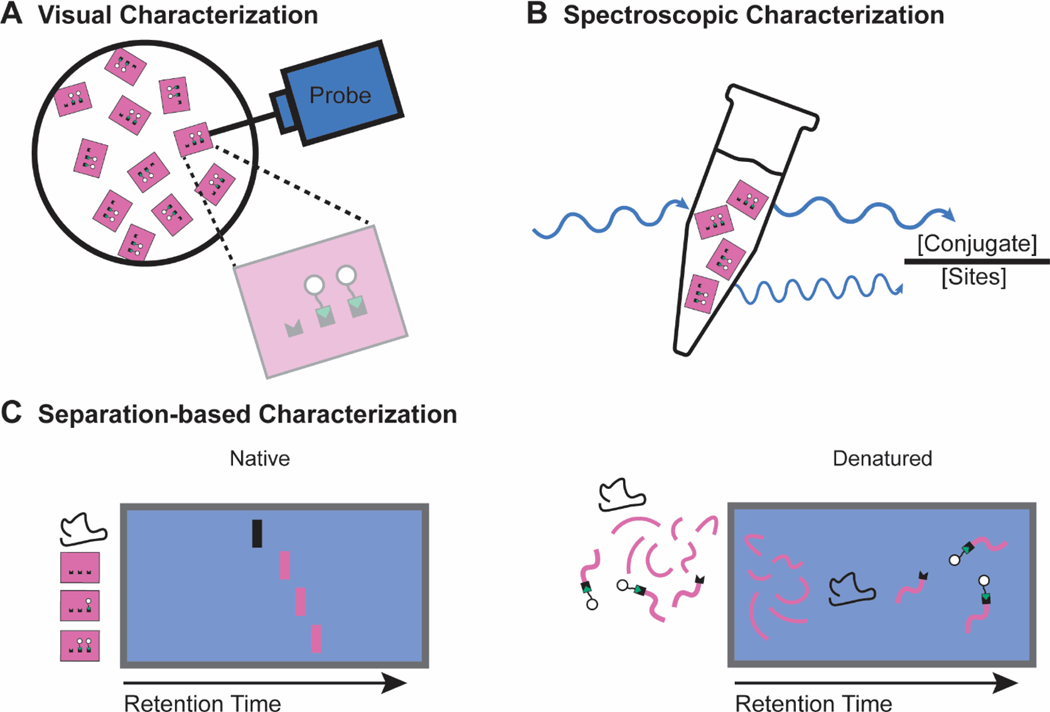
There are three general classes of practical characterization for functionalized DNA origami: visual characterization, spectroscopic characterization, and separation-based characterization (Figure 5).
Figure 5. Characterization techniques used to quantify the functionalization of DNA origami nanostructures.
a | Visual characterization techniques rely on a physical probe (light or matter) to create a visual reconstruction of the nanostructure. The images are then used to quantify functionalization efficiency. b | Spectroscopic characterization techniques rely on a spectral fingerprint of the conjugate. UV or fluorescence spectroscopy determines the concentration of conjugate in a sample, which is compared to the concentration of nanostructures for functionalization efficiency quantification. c | Native separation-based characterization utilizes differences in retention time of functionalized nanostructures and unfunctionalized nanostructures to resolve, and in some cases quantify, functionalization efficiency. Denatured separation-based characterization relies on the physical separation and subsequent identification of the nanostructure components. Comparing the amount of reacted and unreacted functionalization sites allows for functionalization efficiency quantification.
Visual characterization
In visual characterization, a probe generates images of the nanostructures that are then analyzed to count the number of conjugates on the nanostructures (Figure 5a). Early in the development of DNA origami, which began with 2D and bricklike assemblies, atomic force microscopy (AFM) was the standard characterization tool given its ease of use. Thus, AFM was also adopted to characterize nanostructure functionalization, where functionalization sites are visualized through differences in height from the nanostructure. The earliest reports of functionalizing DNA origami with biological conjugates210,211,225, covalent reactions on DNA origami264, and covalent protein functionalization253 relied on AFM to verify the functionalized structure. It has since been extensively used to quantify functionalization with proteins34,37–39,47,58,104,116,119–121,125,128,130,135,137,139,142,166,185,205,207,217,218,224,254,255,257,258,260,261,263 as well as metals201 and nucleic acids176,181.
Transmission electron microscopy (TEM) can also characterize functionalized nanostructures by visualizing the functionalization sites via the difference in contrast between the DNA nanostructure and conjugate. It can quantify protein59,79,131,148,206,209,212,214, metal59,122,150,198,202, and lipid191 functionalization.
Both visual characterization methods determine structural information about the nanostructure and conjugates, include single-particle information not easily attainable by other methods, and require low sample amounts. However, they, have important limitations46,283: they are insensitive to small molecule conjugates such as fluorophores or peptides, have inherent resolution limits, and cannot easily measure the functionalization of 3D nanostructures. Additionally, these techniques are low throughput, limiting their ability to characterize libraries of nanostructures.
Spectroscopic characterization
Spectroscopic characterization relies on ratiometric analysis of the number of conjugates and number of nanostructures in the sample, as determined by spectroscopic fingerprints (Figure 5b). In a typical UV or fluorescence spectroscopy-based experiment, the conjugate of interest either inherently has a spectroscopic fingerprint (either ultraviolet (UV) absorbance or fluorescence) or is engineered to have such a fingerprint (usually by attaching a fluorophore). The conjugate concentration is determined via an external standard, and the DNA nanostructure concentration is measured directly through UV absorbance measurements. The ratio of conjugate concentration to nanostructure concentration gives the functionalization efficiency. Spectroscopic techniques have quantified the functionalization of nucleic acids44,45,182, peptides44,45, fluorophores271, small molecules252, and proteins32,33,43,61,73,89,117,141,144,271 on nanostructures.
Spectroscopic characterization can be implemented simply if the conjugate of interest has an inherent spectroscopic fingerprint (for example, fluorescent tryptophan residues in proteins). However, this technique also suffers from several drawbacks. Many conjugates of interest in biological applications, including most small molecule targeting ligands, have no spectroscopic fingerprint; incorporating one, such as a fluorophore, though commonly performed, can change the biophysical and biochemical nature of the conjugate. Using fluorophores can also be problematic as commonly used cyanine dyes have DNA sequence-dependent fluorescent brightening and quenching285. In addition, the DNA nanostructure concentration measurement is approximate, as molar extinction coefficients are empirical estimates, and researchers often vary in how they measure this concentration. Nanostructure concentration measurements can be improved by incorporating reference fluorophores into the nanostructure prior to assembly, improving the accuracy of nanostructure concentration measurements,79 or by using quantitative polymerase chain reaction assays to provide an unbiased, absolute measure of total nanostructure amounts71. Finally, these ratiometric methods are inaccurate in the presence of conjugate impurities that would lead to the over-estimation of the functionalization efficiency.
Separation-based characterization
Separation-based characterization relies on the physical separation of nanostructures to determine how many sites were functionalized (Figure 5c). Native agarose gel electrophoresis (AGE), a common method to characterize the self-assembly of DNA origami, has also been used to assess DNA nanostructure functionalization with proteins80,115,118,132,149,152,259. The nanostructure band may shift when partially or fully functionalized, depending on conjugate charge, size, and functionalization density. Although an intrinsically qualitative assay, the use of an external reference can produce semi-quantitative results. An advantage of native AGE is that it requires low sample amount, similar to AFM and TEM. It is also simple to implement and can be applied as an initial characterization tool when assessing sample quality, or to screen for optimal reaction conditions. Once an external reference is established, we find this technique especially useful for quality control when producing multiple batches of sample material for preclinical in vivo investigations.
Some separation-based methods use denatured nanostructures to quantitatively determine functionalization efficiencies. After functionalization, the DNA nanostructure is denatured into its scaffold and staple strands, which contain conjugation sites that may or may not be successfully functionalized. Electrophoresis and chromatography separate the conjugated and unconjugated staples from each other and the rest of the DNA nanostructure components via differences in retention times, enabling the functionalization efficiency to be quantified. Electrophoresis-based assays rely on differences between the size and charge of nanostructure components; they were able to quantify functionalization efficiency with small molecule286, and protein123, and peptide conjugates187. In contrast, chromatography-based assays rely on differences in hydrophobicity between nanostructure components. Our group reported a method based on liquid chromatography that affords quantitative functionalization efficiency determination271. Functionalized nanostructures are denatured on a liquid chromatography column, and conjugated staples separate from both non-conjugated functionalization site staples and the rest of the nanostructure components through differences in hydrophobicity to facilitate analysis. We used this technique to monitor the functionalization efficiency of a model DNA origami nanostructure with proteins, peptides, polymers, carbohydrates, and fluorophores. This technique is agnostic to conjugate type and purity, and can be extended to non-origami DNA nanostructures. Electrophoresis- or chromatography-based methods may have better resolution of conjugated and non-conjugated staples, depending on conjugate type and functionalization method employed.
Considerations and analysis
This functionalization efficiency analysis is often skipped when pre-assembly functionalization methods are used, because it is assumed that all staples with desired conjugates are incorporated into the nanostructure. This assumption may not be accurate, however, as staple incorporation into bricklike origami nanostructures is often less than 100%287; it is currently unknown how wireframe objects perform in this regard. The scarcity of facile techniques to determine staple incorporation also contributes to the lack of characterization.
Combining multiple characterization methods to confirm ensemble measurements of functionalization efficiency together with single particle information about nanostructure integrity and population distribution will give the most descriptive information about a functionalized DNA origami nanostructure. When fabricating materials libraries, assay throughput is an additional factor to consider; spectroscopic and separation-based characterization are positioned to handle these high-throughput requirements. Finally, the possibility of nanostructures folding without the entire set of staples287 can become a confounding factor in characterizing functionalized nanostructures.
Almost all characterization of functionalized DNA origami investigates functionalization efficiency. An additional, crucial aspect of their functionalization is ensuring that the attached conjugate is still bioactive, rather than only present. For example, before introducing a delivery vehicle into animal models, it is expected that the attached targeting ligand’s functionality will have been characterized using an in vitro assay to ensure native receptor recognition. B cell receptor32 and T cell receptor89 reporter lines have been used to do just this, and we believe that this type of functional characterization will be an essential and eventually routine step in the fabrication pipeline of functionalized DNA origami in the future.
Outlook
The past 16 years have witnessed substantial advances in what DNA origami can be functionalized with, how DNA origami can be functionalized, and how functionalized DNA origami can be purified and characterized. With these functionalized nanostructures, researchers have conducted impactful investigations in therapeutic delivery, immunology, vaccinology, biological catalysis, and molecular biophysics.
Interesting trends are observed from the history of DNA origami publications in these areas (Supplementary Figure 1, Supplementary Methods), which we analyze below. Now that several bottlenecks in the design and fabrication of DNA origami nanostructures have been overcome, we anticipate that more studies functionalizing DNA origami nanostructures to investigate biological systems will disseminate. It will be interesting to note whether the historically dominant methods in this area will continue to be used, or whether less common techniques will emerge as more powerful methods for specific applications.
Functionalization
Proteins and fluorophores are the dominant conjugates of interest in these fields, and in the past five years, nucleic acids, such as siRNAs, have been increasing in popularity (Supplementary Figure 1a). The post-assembly DNA-DNA hybridization method outpaced the initially popular pre-assembly and post-assembly biotin-streptavidin methods to become the most common functionalization method — presumably owing to its facile implementation (Supplementary Figure 1b). Post-assembly covalent functionalization methods still lag in development and adoption.
From the available reaction details in the literature, many hybridization protocols (Supplementary Figure 2a) operate in the high functionalization efficiency, low equivalents zone, whereas covalent protocols often require large conjugate equivalents to obtain high functionalization efficiency. It appears that the chosen conjugation method, not the conjugate type, determines the functionalization efficiency and conjugate equivalents required (Supplementary Figure 2b). Depending on the method, DNA origami can be functionalized with protein with high or low functionalization efficiency, and the conjugate equivalents required to achieve these conversions span orders of magnitude. Visualizing reported functionalization efficiency and conjugate equivalents over time shows that these important metrics are trending slightly towards improvement, but there is still room for progress (Supplementary Figure 2c and Supplementary Figure 2d). We note that using fewer conjugate equivalents is not only economically desirable; conjugates like proteins have upper concentration limits that restrict the amount of excess conjugate that can be dissolved into the reaction mixture.
We believe there are several factors hindering future progress in functionalization, including insufficient rigor in reporting and characterizing functionalization reactions, the use of unique nanostructures and conjugates by different research groups, and a lack of systematic studies on functionalizing DNA origami across different nanostructure and conjugate types.
Of the publications we analyzed (Supplementary Figure 2), one-quarter did not include information on the equivalents of conjugates used, and one-half did not include information on functionalization efficiency. Though we recognize that this information may not be necessary for accomplishing immediate experimental goals, we posit that reporting detailed procedures and characterization protocols may help to promote development in the field and facilitate reproducibility of experimental results.
Researchers can not only improve existing functionalization methodologies, but also devise new ones. Drawing inspiration from a strategy that incorporates both DNA-protein binding and covalent methods205,255,257, one can envision other approaches to template catalyzed covalent functionalization to improve reaction rates and conversion. To build on progress made in orthogonal covalent reactions of protein conjugates253,255, researchers could extend orthogonal covalent reactions to other conjugate systems, such as by implementing orthogonal click chemistries like thiol-X, Diels-Alder, and tetrazine chemistries to complement the established alkyne-azide chemistries.
Purification
Membrane-based methods have been and still are the most common purification technique, followed by size-based, sedimentation-based, and then affinity-based methods (Supplementary Figure 1c). Although affinity-based magnetic purification has the potential to be a universal purification technique131, it is currently utilized infrequently.
Additional development of purification methodologies may be beneficial for translational prospects. Within membrane methods, centrifugal filtration is the most common technique to purify these functionalized nanostructures. As therapeutic investigations require larger scales, dialysis—which is more scalable than centrifugation-based methods—should be thoroughly investigated as an alternative membrane purification method.
Following up on the earlier comparative analysis of two conjugate types and seven purification techniques131, purification methods should be evaluated over additional parameter spaces: broad conjugate scope, purification recovery yields at different scales of fabrication, and different assembly types such as brick and wireframe structures. Such a systematic study could help researchers identify which purification method will be optimal for their individual investigation.
Finally, all current purification methods focus on separating excess conjugates from functionalized DNA nanostructures. An outstanding, and formidable, technical challenge is how to purify fully functionalized from partially functionalized DNA origami nanostructures.
Characterization
The visual characterization techniques, AFM and TEM, continue to be by far the dominant methods to characterize functionalized DNA origami; there is slow growth in adoption of spectroscopic and separation-based methods (Supplementary Figure 1d). As more of the DNA origami nanostructures used in biophysical or cargo delivery investigations become three-dimensional and functionalized with small molecules, these techniques may no longer be amenable. Spectroscopic and separation-based techniques should be further adopted and advanced to enable more thoroughly characterized systems.
Adapting other techniques may offer additional tools for characterizing functionalization efficiency. Cryogenic electron microscopy (Cryo-EM) is capable of resolving fine structural details of DNA origami objects. For example, recently cryo-EM successfully resolved DNA origami nanostructures functionalized with a poly(Lys)-PEG copolymer, revealing differences between the functionalized and native forms of the nanostructure288. However, cryo-EM is seldom used to characterize functionalized DNA origami. Challenges include the high level of technical expertise needed, advanced instrumentation access, large material amounts and concentrations required, and sufficient contrast of functionalized conjugates. Even in ideal cases, site-specific defects may be difficult to characterize due to limited resolution, and sample averaging performed for reconstruction limits insight into heterogeneous structural features. Applying this advanced technical capability to functionalized DNA origami systems may eventually offer useful insight into how functionalization changes both native nanostructures and conjugates.
Additionally, mass spectrometry, either at the molecular or nanostructure289 levels, could enable new ways to quantify functionalization efficiency. Finally, chemical and related footprinting techniques, applicable to both DNA290 and RNA291,292, may offer new inroads to study functionalized nanostructures.
Final Remarks
Major progress in fabrication methods have enabled DNA origami nanostructures to become a state-of-the-art material platform for probing biological systems. Seminal contributions to the field have expanded the scope of conjugates that can be attached to DNA origami nanostructures, increased the efficiency and effectiveness of these conjugations, developed and scaled purification methods, and developed quantitative and rapid characterization methods. As DNA origami trends towards clinical translation, we believe an emphasis on efficient reaction conditions that achieve quantitative functionalization efficiency; scalable, high-yield purification; and multiplexed, quantitative characterization of functionalized DNA nanostructures will enable this powerful nanotechnology to meet its full potential.
Supplementary Material
Acknowledgements
G.A.K., E-C.W., and M.B. were supported by NIH R01-Al162307, NIH R21-EB026008-S1, ONR N00014-21-1-4013, ONR N00014-20-1-2084, NSF CCF-1956054, ARO ISN W911NF-13-D-0001, and ARO ICB Subaward KK1954. G.A.K. was additionally supported by the National Science Foundation under a Graduate Research Fellowship 2389237.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hong F, Zhang F, Liu Y & Yan H DNA Origami: Scaffolds for Creating Higher Order Structures. Chem Rev 117, 12584–12640, doi: 10.1021/acs.chemrev.6b00825 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Rothemund PW Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302, doi: 10.1038/nature04586 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Douglas SM et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418, doi: 10.1038/nature08016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz H, Douglas SM & Shih WM Folding DNA into twisted and curved nanoscale shapes. Science 325, 725–730, doi: 10.1126/science.1174251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke Y, Ong LL, Shih WM & Yin P Three-dimensional structures self-assembled from DNA bricks. Science 338, 1177–1183, doi: 10.1126/science.1227268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson E et al. DNA rendering of polyhedral meshes at the nanoscale. Nature 523, 441–444, doi: 10.1038/nature14586 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Veneziano R et al. Designer nanoscale DNA assemblies programmed from the top down. Science 352, 1534, doi: 10.1126/science.aaf4388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tikhomirov G, Petersen P & Qian L Fractal assembly of micrometre-scale DNA origami arrays with arbitrary patterns. Nature 552, 67–71, doi: 10.1038/nature24655 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Wagenbauer KF, Sigl C & Dietz H Gigadalton-scale shape-programmable DNA assemblies. Nature 552, 78–83, doi: 10.1038/nature24651 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Yao G et al. Meta-DNA structures. Nat Chem 12, 1067–1075, doi: 10.1038/s41557-020-0539-8 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Huang CM, Kucinic A, Johnson JA, Su HJ & Castro CE Integrated computer-aided engineering and design for DNA assemblies. Nat Mater, doi: 10.1038/s41563-021-00978-5 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Jun H et al. Autonomously designed free-form 2D DNA origami. Sci Adv 5, eaav0655, doi: 10.1126/sciadv.aav0655 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun H et al. Automated Sequence Design of 3D Polyhedral Wireframe DNA Origami with Honeycomb Edges. ACS Nano 13, 2083–2093, doi: 10.1021/acsnano.8b08671 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jun H, Wang X, Bricker WP & Bathe M Automated sequence design of 2D wireframe DNA origami with honeycomb edges. Nat Commun 10, 5419, doi: 10.1038/s41467-019-13457-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun H et al. Rapid prototyping of arbitrary 2D and 3D wireframe DNA origami. Nucleic Acids Res 49, 10265–10274, doi: 10.1093/nar/gkab762 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veneziano R et al. In vitro synthesis of gene-length single-stranded DNA. Sci Rep 8, 6548, doi: 10.1038/s41598-018-24677-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nafisi PM, Aksel T & Douglas SM Construction of a novel phagemid to produce custom DNA origami scaffolds. Synth Biol (Oxf) 3, doi: 10.1093/synbio/ysy015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd TR, Du RR, Huang H, Wamhoff EC & Bathe M Bioproduction of pure, kilobase-scale single-stranded DNA. Sci Rep 9, 6121, doi: 10.1038/s41598-019-42665-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelhardt FAS et al. Custom-Size, Functional, and Durable DNA Origami with Design-Specific Scaffolds. ACS Nano 13, 5015–5027, doi: 10.1021/acsnano.9b01025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minev D et al. Rapid in vitro production of single-stranded DNA. Nucleic Acids Res 47, 11956–11962, doi: 10.1093/nar/gkz998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noteborn WEM, Abendstein L & Sharp TH One-Pot Synthesis of Defined-Length ssDNA for Multiscaffold DNA Origami. Bioconjugate Chem 32, 94–98, doi: 10.1021/acs.bioconjchem.0c00644 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Praetorius F et al. Biotechnological mass production of DNA origami. Nature 552, 84–87, doi: 10.1038/nature24650 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Ducani C, Kaul C, Moche M, Shih WM & Hogberg B Enzymatic production of ‘monoclonal stoichiometric’ single-stranded DNA oligonucleotides. Nat Methods 10, 647–652, doi: 10.1038/nmeth.2503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt TL et al. Scalable amplification of strand subsets from chip-synthesized oligonucleotide libraries. Nat Commun 6, 8634, doi: 10.1038/ncomms9634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halley PD, Patton RA, Chowdhury A, Byrd JC & Castro CE Low-cost, simple, and scalable self-assembly of DNA origami nanostructures. Nano Res 12, 1207–1215, doi: 10.1007/s12274-019-2384-x (2019). [DOI] [Google Scholar]
- 26.Afonin KA, Dobrovolskaia MA, Church G & Bathe M Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology. ACS Nano 14, 9221–9227, doi: 10.1021/acsnano.0c04753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrovoskaia MA & Bathe M Opportunities and challenges for the clinical translation of structuredDNAassemblies as gene therapeutic delivery and vaccine vectors. Wires Nanomed Nanobi 13, e1657, doi: 10.1002/wnan.1657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afonin KA, Dobrovolskaia MA, Ke W, Grodzinski P & Bathe M Critical review of nucleic acid nanotechnology to identify gaps and inform a strategy for accelerated clinical translation. Adv Drug Deliv Rev, 114081, doi: 10.1016/j.addr.2021.114081 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujold KE, Lacroix A & Sleiman HF DNA Nanostructures at the Interface with Biology. Chem-Us 4, 495–521, doi: 10.1016/j.chempr.2018.02.005 (2018). [DOI] [Google Scholar]
- 30.Wamhoff EC et al. Programming Structured DNA Assemblies to Probe Biophysical Processes. Annu Rev Biophys 48, 395–419, doi: 10.1146/annurev-biophys-052118-115259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacca B & Niemeyer CM DNA origami: the art of folding DNA. Angew Chem Int Ed Engl 51, 58–66, doi: 10.1002/anie.201105846 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Veneziano R et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat Nanotechnol 15, 716–723, doi: 10.1038/s41565-020-0719-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellmeier J et al. DNA origami demonstrate the unique stimulatory power of single pMHCs as T cell antigens. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2016857118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang T et al. Spatial Regulation of T-Cell Signaling by Programmed Death-Ligand 1 on Wireframe DNA Origami Flat Sheets. ACS Nano 15, 3441–3452, doi: 10.1021/acsnano.0c10632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern N, Dong R, Douglas SM, Vale RD & Morrissey MA Tight nanoscale clustering of Fcgamma receptors using DNA origami promotes phagocytosis. Elife 10, doi: 10.7554/eLife.68311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong R et al. DNA origami patterning of synthetic T cell receptors reveals spatial control of the sensitivity and kinetics of signal activation. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2109057118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosier B et al. Proximity-induced caspase-9 activation on a DNA origami-based synthetic apoptosome. Nat Catal 3, 295–306, doi: 10.1038/s41929-019-0403-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein WP et al. Enhanced Catalysis from Multienzyme Cascades Assembled on a DNA Origami Triangle. ACS Nano 13, 13677–13689, doi: 10.1021/acsnano.9b05746 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Wang D et al. An Addressable 2D Heterogeneous Nanoreactor to Study the Enzyme-Catalyzed Reaction at the Interface. Small 13, doi: 10.1002/smll.201700594 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Yang YR et al. 2D Enzyme Cascade Network with Efficient Substrate Channeling by Swinging Arms. Chembiochem 19, 212–216, doi: 10.1002/cbic.201700613 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Shaw A et al. Binding to nanopatterned antigens is dominated by the spatial tolerance of antibodies. Nat Nanotechnol 14, 184–190, doi: 10.1038/s41565-018-0336-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas SM, Bachelet I & Church GM A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834, doi: 10.1126/science.1214081 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Li S et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol 36, 258–264, doi: 10.1038/nbt.4071 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Wang Z et al. A Tubular DNA Nanodevice as a siRNA/Chemo-Drug Co-delivery Vehicle for Combined Cancer Therapy. Angew Chem Int Ed Engl 60, 2594–2598, doi: 10.1002/anie.202009842 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Liu S et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater 20, 421–430, doi: 10.1038/s41563-020-0793-6 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Dey S et al. DNA origami. Nature Reviews Methods Primers 1, 13, doi: 10.1038/s43586-020-00009-8 (2021). [DOI] [Google Scholar]
- 47.Angelin A et al. Multiscale Origami Structures as Interface for Cells. Angew Chem Int Ed Engl 54, 15813–15817, doi: 10.1002/anie.201509772 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Bastings MMC et al. Modulation of the Cellular Uptake of DNA Origami through Control over Mass and Shape. Nano Lett 18, 3557–3564, doi: 10.1021/acs.nanolett.8b00660 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Burns JR, Lamarre B, Pyne ALB, Noble JE & Ryadnov MG DNA Origami Inside-Out Viruses. ACS Synth Biol 7, 767–773, doi: 10.1021/acssynbio.7b00278 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Czogalla A et al. Amphipathic DNA origami nanoparticles to scaffold and deform lipid membrane vesicles. Angew Chem Int Ed Engl 54, 6501–6505, doi: 10.1002/anie.201501173 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Czogalla A, Kauert DJ, Seidel R, Schwille P & Petrov EP DNA origami nanoneedles on freestanding lipid membranes as a tool to observe isotropic-nematic transition in two dimensions. Nano Lett 15, 649–655, doi: 10.1021/nl504158h (2015). [DOI] [PubMed] [Google Scholar]
- 52.Deng Y et al. Intracellular Delivery of Nanomaterials via an Inertial Microfluidic Cell Hydroporator. Nano Lett 18, 2705–2710, doi: 10.1021/acs.nanolett.8b00704 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Fu M et al. Observation of intracellular interactions between DNA origami and lysosomes by the fluorescence localization method. Chem Commun (Camb) 52, 9240–9242, doi: 10.1039/c6cc00484a (2016). [DOI] [PubMed] [Google Scholar]
- 54.Funke JJ, Ketterer P, Lieleg C, Korber P & Dietz H Exploring Nucleosome Unwrapping Using DNA Origami. Nano Lett 16, 7891–7898, doi: 10.1021/acs.nanolett.6b04169 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Funke JJ et al. Uncovering the forces between nucleosomes using DNA origami. Sci Adv 2, e1600974, doi: 10.1126/sciadv.1600974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grome MW, Zhang Z, Pincet F & Lin C Vesicle Tubulation with Self-Assembling DNA Nanosprings. Angew Chem Int Ed Engl 57, 5330–5334, doi: 10.1002/anie.201800141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirtz M, Brglez J, Fuchs H & Niemeyer CM Selective Binding of DNA Origami on Biomimetic Lipid Patches. Small 11, 5752–5758, doi: 10.1002/smll.201501333 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Hu Y, Dominguez CM, Christ S & Niemeyer CM Postsynthetic Functionalization of DNA-Nanocomposites with Proteins Yields Bioinstructive Matrices for Cell Culture Applications. Angew Chem Int Ed Engl 59, 19016–19020, doi: 10.1002/anie.202008471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ijas H, Hakaste I, Shen B, Kostiainen MA & Linko V Reconfigurable DNA Origami Nanocapsule for pH-Controlled Encapsulation and Display of Cargo. ACS Nano 13, 5959–5967, doi: 10.1021/acsnano.9b01857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jorge AF, Avino A, Pais A, Eritja R & Fabrega C DNA-based nanoscaffolds as vehicles for 5-fluoro-2’-deoxyuridine oligomers in colorectal cancer therapy. Nanoscale 10, 7238–7249, doi: 10.1039/c7nr08442k (2018). [DOI] [PubMed] [Google Scholar]
- 61.Jusuk I, Vietz C, Raab M, Dammeyer T & Tinnefeld P Super-Resolution Imaging Conditions for enhanced Yellow Fluorescent Protein (eYFP) Demonstrated on DNA Origami Nanorulers. Sci Rep 5, 14075, doi: 10.1038/srep14075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khmelinskaia A, Mucksch J, Petrov EP, Franquelim HG & Schwille P Control of Membrane Binding and Diffusion of Cholesteryl-Modified DNA Origami Nanostructures by DNA Spacers. Langmuir 34, 14921–14931, doi: 10.1021/acs.langmuir.8b01850 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Kocabey S et al. Cellular Uptake of Tile-Assembled DNA Nanotubes. Nanomaterials (Basel) 5, 47–60, doi: 10.3390/nano5010047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosuri P, Altheimer BD, Dai M, Yin P & Zhuang X Rotation tracking of genome-processing enzymes using DNA origami rotors. Nature 572, 136–140, doi: 10.1038/s41586-019-1397-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kramm K et al. DNA origami-based single-molecule force spectroscopy elucidates RNA Polymerase III pre-initiation complex stability. Nat Commun 11, 2828, doi: 10.1038/s41467-020-16702-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.List J, Weber M & Simmel FC Hydrophobic actuation of a DNA origami bilayer structure. Angew Chem Int Ed Engl 53, 4236–4239, doi: 10.1002/anie.201310259 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Liu J et al. A Tailored DNA Nanoplatform for Synergistic RNAi-/Chemotherapy of Multidrug-Resistant Tumors. Angew Chem Int Ed Engl 57, 15486–15490, doi: 10.1002/anie.201809452 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Maezawa T et al. DNA density-dependent uptake of DNA origami-based two-or three-dimensional nanostructures by immune cells. Nanoscale 12, 14818–14824, doi: 10.1039/d0nr02361b (2020). [DOI] [PubMed] [Google Scholar]
- 69.Mathur D & Henderson ER Programmable DNA Nanosystem for Molecular Interrogation. Sci Rep 6, 27413, doi: 10.1038/srep27413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohtsuki S et al. Folding of single-stranded circular DNA into rigid rectangular DNA accelerates its cellular uptake. Nanoscale 11, 23416–23422, doi: 10.1039/c9nr08695a (2019). [DOI] [PubMed] [Google Scholar]
- 71.Okholm AH et al. Quantification of cellular uptake of DNA nanostructures by qPCR. Methods 67, 193–197, doi: 10.1016/j.ymeth.2014.01.013 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Selnihhin D, Sparvath SM, Preus S, Birkedal V & Andersen ES Multifluorophore DNA Origami Beacon as a Biosensing Platform. ACS Nano 12, 5699–5708, doi: 10.1021/acsnano.8b01510 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Verma V et al. Using Protein Dimers to Maximize the Protein Hybridization Efficiency with Multisite DNA Origami Scaffolds. PLoS One 10, e0137125, doi: 10.1371/journal.pone.0137125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu S et al. A Quick-responsive DNA Nanotechnology Device for Bio-molecular Homeostasis Regulation. Sci Rep 6, 31379, doi: 10.1038/srep31379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing C et al. Spatial Regulation of Biomolecular Interactions with a Switchable Trident-Shaped DNA Nanoactuator. ACS Appl Mater Interfaces 10, 32579–32587, doi: 10.1021/acsami.8b10761 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Zadegan RM & Hughes WL CAGE: Chromatin Analogous Gene Expression. ACS Synth Biol 6, 1800–1806, doi: 10.1021/acssynbio.7b00045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H et al. DNA nanostructures coordinate gene silencing in mature plants. Proc Natl Acad Sci U S A 116, 7543–7548, doi: 10.1073/pnas.1818290116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaffert DH et al. Intracellular Delivery of a Planar DNA Origami Structure by the Transferrin-Receptor Internalization Pathway. Small 12, 2634–2640, doi: 10.1002/smll.201503934 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Kohman RE, Cha SS, Man HY & Han X Light-Triggered Release of Bioactive Molecules from DNA Nanostructures. Nano Lett 16, 2781–2785, doi: 10.1021/acs.nanolett.6b00530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Journot CMA, Ramakrishna V, Wallace MI & Turberfield AJ Modifying Membrane Morphology and Interactions with DNA Origami Clathrin-Mimic Networks. ACS Nano 13, 9973–9979, doi: 10.1021/acsnano.8b07734 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silvester E et al. DNA origami signposts for identifying proteins on cell membranes by electron cryotomography. Cell 184, 1110–1121 e1116, doi: 10.1016/j.cell.2021.01.033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwabuchi S, Kawamata I, Murata S & Nomura SM A large, square-shaped, DNA origami nanopore with sealing function on a giant vesicle membrane. Chem Commun (Camb) 57, 2990–2993, doi: 10.1039/d0cc07412h (2021). [DOI] [PubMed] [Google Scholar]
- 83.Wang Y et al. DNA Origami Penetration in Cell Spheroid Tissue Models is Enhanced by Wireframe Design. Adv Mater 33, e2008457, doi: 10.1002/adma.202008457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu X et al. An RNA/DNA hybrid origami-based nanoplatform for efficient gene therapy. Nanoscale 13, 12848–12853, doi: 10.1039/d1nr00517k (2021). [DOI] [PubMed] [Google Scholar]
- 85.Pal S & Rakshit T Folate-Functionalized DNA Origami for Targeted Delivery of Doxorubicin to Triple-Negative Breast Cancer. Front Chem 9, 721105, doi: 10.3389/fchem.2021.721105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smolkova B et al. Protein Corona Inhibits Endosomal Escape of Functionalized DNA Nanostructures in Living Cells. ACS Appl Mater Interfaces 13, 46375–46390, doi: 10.1021/acsami.1c14401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomsen RP et al. A large size-selective DNA nanopore with sensing applications. Nat Commun 10, 5655, doi: 10.1038/s41467-019-13284-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang H et al. Amphiphilic and Biocompatible DNA Origami-Based Emulsion Formation and Nanopore Release for Anti-Melanogenesis Therapy. Small 17, e2104831, doi: 10.1002/smll.202104831 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Hellmeier J et al. Strategies for the Site-Specific Decoration of DNA Origami Nanostructures with Functionally Intact Proteins. ACS Nano 15, 15057–15068, doi: 10.1021/acsnano.1c05411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bell NA & Keyser UF Digitally encoded DNA nanostructures for multiplexed, single-molecule protein sensing with nanopores. Nat Nanotechnol 11, 645–651, doi: 10.1038/nnano.2016.50 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Brglez J, Ahmed I & Niemeyer CM Photocleavable ligands for protein decoration of DNA nanostructures. Org Biomol Chem 13, 5102–5104, doi: 10.1039/c5ob00316d (2015). [DOI] [PubMed] [Google Scholar]
- 92.Iwaki M, Wickham SF, Ikezaki K, Yanagida T & Shih WM A programmable DNA origami nanospring that reveals force-induced adjacent binding of myosin VI heads. Nat Commun 7, 13715, doi: 10.1038/ncomms13715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kielar C et al. Pharmacophore Nanoarrays on DNA Origami Substrates as a Single-Molecule Assay for Fragment-Based Drug Discovery. Angew Chem Int Ed Engl 57, 14873–14877, doi: 10.1002/anie.201806778 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Kuzuya A et al. Allosteric control of nanomechanical DNA origami pinching devices for enhanced target binding. Chem Commun (Camb) 53, 8276–8279, doi: 10.1039/c7cc03991c (2017). [DOI] [PubMed] [Google Scholar]
- 95.Zhang P et al. Capturing transient antibody conformations with DNA origami epitopes. Nat Commun 11, 3114, doi: 10.1038/s41467-020-16949-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang P et al. Quantitative Measurement of Spatial Effects of DNA Origami on Molecular Binding Reactions Detected using Atomic Force Microscopy. ACS Appl Mater Interfaces 11, 21973–21981, doi: 10.1021/acsami.9b01691 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Liu Y et al. The effects of overhang placement and multivalency on cell labeling by DNA origami. Nanoscale 13, 6819–6828, doi: 10.1039/d0nr09212f (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wijesekara P et al. Accessing and Assessing the Cell-Surface Glycocalyx Using DNA Origami. Nano Lett 21, 4765–4773, doi: 10.1021/acs.nanolett.1c01236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffecker IT et al. Solution-Controlled Conformational Switching of an Anchored Wireframe DNA Nanostructure. Small 15, e1803628, doi: 10.1002/smll.201803628 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Eklund AS, Comberlato A, Parish IA, Jungmann R & Bastings MM C. Quantification of Strand Accessibility in Biostable DNA Origami with Single-Staple Resolution. ACS Nano, doi: 10.1021/acsnano.1c05540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu K, Xu C & Liu J Regulation of cell binding and entry by DNA origami mediated spatial distribution of aptamers. J Mater Chem B 8, 6802–6809, doi: 10.1039/d0tb00663g (2020). [DOI] [PubMed] [Google Scholar]
- 102.Torelli E et al. Cotranscriptional Folding of a Bio-orthogonal Fluorescent Scaffolded RNA Origami. ACS Synth Biol 9, 1682–1692, doi: 10.1021/acssynbio.0c00009 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Lu Z, Wang Y, Xu D & Pang L Aptamer-tagged DNA origami for spatially addressable detection of aflatoxin B1. Chem Commun (Camb) 53, 941–944, doi: 10.1039/c6cc08831g (2017). [DOI] [PubMed] [Google Scholar]
- 104.Zhao S et al. Efficient Intracellular Delivery of RNase A Using DNA Origami Carriers. ACS Appl Mater Interfaces 11, 11112–11118, doi: 10.1021/acsami.8b21724 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Numajiri K, Yamazaki T, Kimura M, Kuzuya A & Komiyama M Discrete and active enzyme nanoarrays on DNA origami scaffolds purified by affinity tag separation. J Am Chem Soc 132, 9937–9939, doi: 10.1021/ja104702q (2010). [DOI] [PubMed] [Google Scholar]
- 106.Udomprasert A et al. Amyloid fibrils nucleated and organized by DNA origami constructions. Nat Nanotechnol 9, 537–541, doi: 10.1038/nnano.2014.102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sorensen RS et al. Enzymatic ligation of large biomolecules to DNA. ACS Nano 7, 8098–8104, doi: 10.1021/nn403386f (2013). [DOI] [PubMed] [Google Scholar]
- 108.Chen X et al. Aptamer-Integrated Scaffolds for Biologically Functional DNA Origami Structures. ACS Appl Mater Interfaces 13, 39711–39718, doi: 10.1021/acsami.1c09307 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Akinc A et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 26, 561–569, doi: 10.1038/nbt1402 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dahlman JE et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci U S A 114, 2060–2065, doi: 10.1073/pnas.1620874114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amir Y et al. Universal computing by DNA origami robots in a living animal. Nat Nanotechnol 9, 353–357, doi: 10.1038/nnano.2014.58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arnon S et al. Thought-Controlled Nanoscale Robots in a Living Host. PLoS One 11, e0161227, doi: 10.1371/journal.pone.0161227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y et al. A Synthetic Light-Driven Substrate Channeling System for Precise Regulation of Enzyme Cascade Activity Based on DNA Origami. J Am Chem Soc 140, 8990–8996, doi: 10.1021/jacs.8b05429 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Derr ND et al. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science 338, 662–665, doi: 10.1126/science.1226734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Driller-Colangelo AR, Chau KW, Morgan JM & Derr ND Cargo rigidity affects the sensitivity of dynein ensembles to individual motor pausing. Cytoskeleton (Hoboken) 73, 693–702, doi: 10.1002/cm.21339 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Feng Y, Hashiya F, Hidaka K, Sugiyama H & Endo M Direct Observation of Dynamic Interactions between Orientation-Controlled Nucleosomes in a DNA Origami Frame. Chemistry 26, 15282–15289, doi: 10.1002/chem.202003071 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Fisher PDE et al. A Programmable DNA Origami Platform for Organizing Intrinsically Disordered Nucleoporins within Nanopore Confinement. ACS Nano 12, 1508–1518, doi: 10.1021/acsnano.7b08044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu J et al. Assembly of multienzyme complexes on DNA nanostructures. Nat Protoc 11, 2243–2273, doi: 10.1038/nprot.2016.139 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Fu Y et al. Single-step rapid assembly of DNA origami nanostructures for addressable nanoscale bioreactors. J Am Chem Soc 135, 696–702, doi: 10.1021/ja3076692 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Fujita K, Ohmachi M, Ikezaki K, Yanagida T & Iwaki M Direct visualization of human myosin II force generation using DNA origami-based thick filaments. Commun Biol 2, 437, doi: 10.1038/s42003-019-0683-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ge Z et al. Constructing Submonolayer DNA Origami Scaffold on Gold Electrode for Wiring of Redox Enzymatic Cascade Pathways. ACS Appl Mater Interfaces 11, 13881–13887, doi: 10.1021/acsami.8b12374 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Grossi G, Dalgaard Ebbesen Jepsen M, Kjems J & Andersen ES Control of enzyme reactions by a reconfigurable DNA nanovault. Nat Commun 8, 992, doi: 10.1038/s41467-017-01072-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hahn J, Chou LYT, Sorensen RS, Guerra RM & Shih WM Extrusion of RNA from a DNA-Origami-Based Nanofactory. ACS Nano 14, 1550–1559, doi: 10.1021/acsnano.9b06466 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Kaminska I, Bohlen J, Mackowski S, Tinnefeld P & Acuna GP Strong Plasmonic Enhancement of a Single Peridinin-Chlorophyll a-Protein Complex on DNA Origami-Based Optical Antennas. ACS Nano 12, 1650–1655, doi: 10.1021/acsnano.7b08233 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Ke G et al. Directional Regulation of Enzyme Pathways through the Control of Substrate Channeling on a DNA Origami Scaffold. Angew Chem Int Ed Engl 55, 7483–7486, doi: 10.1002/anie.201603183 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Ke Y, Meyer T, Shih WM & Bellot G Regulation at a distance of biomolecular interactions using a DNA origami nanoactuator. Nat Commun 7, 10935, doi: 10.1038/ncomms10935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ketterer P et al. DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex. Nat Commun 9, 902, doi: 10.1038/s41467-018-03313-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marth G et al. Precision Templated Bottom-Up Multiprotein Nanoassembly through Defined Click Chemistry Linkage to DNA. ACS Nano 11, 5003–5010, doi: 10.1021/acsnano.7b01711 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Matsuda K et al. Artificial Smooth Muscle Model Composed of Hierarchically Ordered Microtubule Asters Mediated by DNA Origami Nanostructures. Nano Lett 19, 3933–3938, doi: 10.1021/acs.nanolett.9b01201 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Rosier B et al. Incorporation of native antibodies and Fc-fusion proteins on DNA nanostructures via a modular conjugation strategy. Chem Commun (Camb) 53, 7393–7396, doi: 10.1039/c7cc04178k (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shaw A, Benson E & Hogberg B Purification of functionalized DNA origami nanostructures. ACS Nano 9, 4968–4975, doi: 10.1021/nn507035g (2015). [DOI] [PubMed] [Google Scholar]
- 132.Shaw A et al. Spatial control of membrane receptor function using ligand nanocalipers. Nat Methods 11, 841–846, doi: 10.1038/nmeth.3025 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Sommese RF et al. Patterning protein complexes on DNA nanostructures using a GFP nanobody. Protein Sci 25, 2089–2094, doi: 10.1002/pro.3020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stanley GJ et al. Quantification of Biomolecular Dynamics Inside Real and Synthetic Nuclear Pore Complexes Using Time-Resolved Atomic Force Microscopy. ACS Nano 13, 7949–7956, doi: 10.1021/acsnano.9b02424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stephanopoulos N et al. Immobilization and one-dimensional arrangement of virus capsids with nanoscale precision using DNA origami. Nano Lett 10, 2714–2720, doi: 10.1021/nl1018468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun L et al. Real-Time Imaging of Single-Molecule Enzyme Cascade Using a DNA Origami Raft. J Am Chem Soc 139, 17525–17532, doi: 10.1021/jacs.7b09323 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Vogele K, List J, Simmel FC & Pirzer T Enhanced Efficiency of an Enzyme Cascade on DNA-Activated Silica Surfaces. Langmuir 34, 14780–14786, doi: 10.1021/acs.langmuir.8b01770 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Wang D et al. A switchable DNA origami nanochannel for regulating molecular transport at the nanometer scale. Nanoscale 8, 3944–3948, doi: 10.1039/c5nr08206d (2016). [DOI] [PubMed] [Google Scholar]
- 139.Weinhold E & Chakraborty B DNA modification and visualization on an origami-based enzyme nano-factory. Nanoscale 13, 2465–2471, doi: 10.1039/d0nr07618j (2021). [DOI] [PubMed] [Google Scholar]
- 140.Williams ND et al. DNA-Origami-Based Fluorescence Brightness Standards for Convenient and Fast Protein Counting in Live Cells. Nano Lett 20, 8890–8896, doi: 10.1021/acs.nanolett.0c03925 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu W et al. A Programmable DNA Origami Platform to Organize SNAREs for Membrane Fusion. J Am Chem Soc 138, 4439–4447, doi: 10.1021/jacs.5b13107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu Y et al. Single-Molecule Studies of Allosteric Inhibition of Individual Enzyme on a DNA Origami Reactor. J Phys Chem Lett 9, 6786–6794, doi: 10.1021/acs.jpclett.8b02992 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Zanacchi FC et al. A DNA origami platform for quantifying protein copy number in super-resolution. Nat Methods 14, 789–792, doi: 10.1038/nmeth.4342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao Z et al. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat Commun 7, 10619, doi: 10.1038/ncomms10619 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou J et al. A new biochromatography model based on DNA origami assembled PPARgamma: construction and evaluation. Anal Bioanal Chem 409, 3059–3065, doi: 10.1007/s00216-017-0274-1 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Rai A, Vang D, Ritt M & Sivaramakrishnan S Dynamic multimerization of Dab2-Myosin VI complexes regulates cargo processivity while minimizing cortical actin reorganization. J Biol Chem 296, 100232, doi: 10.1074/jbc.RA120.012703 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abe K, Sugiyama H & Endo M Construction of an optically controllable CRISPR-Cas9 system using a DNA origami nanostructure. Chem Commun (Camb) 57, 5594–5596, doi: 10.1039/d1cc00876e (2021). [DOI] [PubMed] [Google Scholar]
- 148.Berger RML et al. Nanoscale FasL Organization on DNA Origami to Decipher Apoptosis Signal Activation in Cells. Small 17, e2101678, doi: 10.1002/smll.202101678 (2021). [DOI] [PubMed] [Google Scholar]
- 149.Cremers GAO et al. Determinants of Ligand-Functionalized DNA Nanostructure-Cell Interactions. J Am Chem Soc 143, 10131–10142, doi: 10.1021/jacs.1c02298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen Q et al. DNA-Origami NanoTrap for Studying the Selective Barriers Formed by Phenylalanine-Glycine-Rich Nucleoporins. J Am Chem Soc 143, 12294–12303, doi: 10.1021/jacs.1c05550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sigl C et al. Programmable icosahedral shell system for virus trapping. Nat Mater 20, 1281–1289, doi: 10.1038/s41563-021-01020-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sprengel A et al. Tailored protein encapsulation into a DNA host using geometrically organized supramolecular interactions. Nat Commun 8, 14472, doi: 10.1038/ncomms14472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Akbari E et al. Engineering Cell Surface Function with DNA Origami. Adv Mater 29, doi: 10.1002/adma.201703632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chopra A, Krishnan S & Simmel FC Electrotransfection of Polyamine Folded DNA Origami Structures. Nano Lett 16, 6683–6690, doi: 10.1021/acs.nanolett.6b03586 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Dai M, Jungmann R & Yin P Optical imaging of individual biomolecules in densely packed clusters. Nat Nanotechnol 11, 798–807, doi: 10.1038/nnano.2016.95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dutta PK et al. Programmable Multivalent DNA-Origami Tension Probes for Reporting Cellular Traction Forces. Nano Lett 18, 4803–4811, doi: 10.1021/acs.nanolett.8b01374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnson-Buck A, Jiang S, Yan H & Walter NG DNA-cholesterol barges as programmable membrane-exploring agents. ACS Nano 8, 5641–5649, doi: 10.1021/nn500108k (2014). [DOI] [PubMed] [Google Scholar]
- 158.Kaplan C et al. Absolute Arrangement of Subunits in Cytoskeletal Septin Filaments in Cells Measured by Fluorescence Microscopy. Nano Lett 15, 3859–3864, doi: 10.1021/acs.nanolett.5b00693 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Mucksch J et al. Quantifying Reversible Surface Binding via Surface-Integrated Fluorescence Correlation Spectroscopy. Nano Lett 18, 3185–3192, doi: 10.1021/acs.nanolett.8b00875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rahman MA et al. Systemic Delivery of Bc12-Targeting siRNA by DNA Nanoparticles Suppresses Cancer Cell Growth. Angew Chem Int Ed Engl 56, 16023–16027, doi: 10.1002/anie.201709485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tohgasaki T et al. A Photocaged DNA Nanocapsule for Controlled Unlocking and Opening inside the Cell. Bioconjug Chem 30, 1860–1863, doi: 10.1021/acs.bioconjchem.9b00040 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Wang P et al. Visualization of the Cellular Uptake and Trafficking of DNA Origami Nanostructures in Cancer Cells. J Am Chem Soc 140, 2478–2484, doi: 10.1021/jacs.7b09024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wiraja C et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun 10, 1147, doi: 10.1038/s41467-019-09029-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ge Z et al. DNA Origami-Enabled Engineering of Ligand-Drug Conjugates for Targeted Drug Delivery. Small 16, e1904857, doi: 10.1002/smll.201904857 (2020). [DOI] [PubMed] [Google Scholar]
- 165.Franquelim HG, Dietz H & Schwille P Reversible membrane deformations by straight DNA origami filaments. Soft Matter 17, 276–287, doi: 10.1039/d0sm00150c (2021). [DOI] [PubMed] [Google Scholar]
- 166.Lin P, Dinh H, Nakata E & Morii T Dynamic Shape Transformation of a DNA Scaffold Applied for an Enzyme Nanocarrier. Front Chem 9, 697857, doi: 10.3389/fchem.2021.697857 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jahnke K et al. Proton gradients from light-harvesting E. coli control DNA assemblies for synthetic cells. Nat Commun 12, 3967, doi: 10.1038/s41467-021-24103-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fragasso A et al. Reconstitution of Ultrawide DNA Origami Pores in Liposomes for Transmembrane Transport of Macromolecules. ACS Nano, doi: 10.1021/acsnano.1c01669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu J et al. Reconstructing Soma-Soma Synapse-like Vesicular Exocytosis with DNA Origami. ACS Cent Sci 7, 1400–1407, doi: 10.1021/acscentsci.1c00645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daems D et al. Controlling the Bioreceptor Spatial Distribution at the Nanoscale for Single Molecule Counting in Microwell Arrays. ACS Sens 4, 2327–2335, doi: 10.1021/acssensors.9b00877 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Pan Q et al. Aptamer-Functionalized DNA Origami for Targeted Codelivery of Antisense Oligonucleotides and Doxorubicin to Enhance Therapy in Drug-Resistant Cancer Cells. ACS Appl Mater Interfaces 12, 400–409, doi: 10.1021/acsami.9b20707 (2020). [DOI] [PubMed] [Google Scholar]
- 172.Raveendran M, Lee AJ, Sharma R, Walti C & Actis P Rational design of DNA nanostructures for single molecule biosensing. Nat Commun 11, 4384, doi: 10.1038/s41467-020-18132-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rutten I, Daems D & Lammertyn J Boosting biomolecular interactions through DNA origami nano-tailored biosensing interfaces. J Mater Chem B 8, 3606–3615, doi: 10.1039/c9tb02439e (2020). [DOI] [PubMed] [Google Scholar]
- 174.Sun PC et al. Site-specific anchoring aptamer C2NP on DNA origami nanostructures for cancer treatment. Rsc Adv 8, 26300–26308, doi: 10.1039/c8ra04589e (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang L et al. An Intelligent DNA Nanorobot for Autonomous Anticoagulation. Angew Chem Int Ed Engl 59, 17697–17704, doi: 10.1002/anie.202007962 (2020). [DOI] [PubMed] [Google Scholar]
- 176.Zhao S et al. A DNA origami-based aptamer nanoarray for potent and reversible anticoagulation in hemodialysis. Nat Commun 12, 358, doi: 10.1038/s41467-020-20638-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu F et al. Profiling and Regulating Proteins That Adsorb to DNA Materials in Human Serum. Anal Chem 93, 8671–8679, doi: 10.1021/acs.analchem.1c02075 (2021). [DOI] [PubMed] [Google Scholar]
- 178.Liu F et al. A tetrahedral DNA nanorobot with conformational change in response to molecular trigger. Nanoscale 13, 15552–15559, doi: 10.1039/d1nr02757c (2021). [DOI] [PubMed] [Google Scholar]
- 179.Zhang T et al. Construction of a reconfigurable DNA nanocage for encapsulating a TMV disk. Chem Commun (Camb) 55, 8951–8954, doi: 10.1039/c9cc03109j (2019). [DOI] [PubMed] [Google Scholar]
- 180.Zhou K, Ke Y & Wang Q Selective in Situ Assembly of Viral Protein onto DNA Origami. J Am Chem Soc 140, 8074–8077, doi: 10.1021/jacs.8b03914 (2018). [DOI] [PubMed] [Google Scholar]
- 181.Zhou K, Zhou Y, Pan V, Wang Q & Ke Y Programming Dynamic Assembly of Viral Proteins with DNA Origami. J Am Chem Soc 142, 5929–5932, doi: 10.1021/jacs.9b13773 (2020). [DOI] [PubMed] [Google Scholar]
- 182.Xu T et al. DNA Origami Frameworks Enabled Self-Protective siRNA Delivery for Dual Enhancement of Chemo-Photothermal Combination Therapy. Small 17, e2101780, doi: 10.1002/smll.202101780 (2021). [DOI] [PubMed] [Google Scholar]
- 183.Buchberger A, Simmons CR, Fahmi NE, Freeman R & Stephanopoulos N Hierarchical Assembly of Nucleic Acid/Coiled-Coil Peptide Nanostructures. J Am Chem Soc 142, 1406–1416, doi: 10.1021/jacs.9b11158 (2020). [DOI] [PubMed] [Google Scholar]
- 184.Hawkes W et al. Probing the nanoscale organisation and multivalency of cell surface receptors: DNA origami nanoarrays for cellular studies with single-molecule control. Faraday Discuss 219, 203–219, doi: 10.1039/c9fd00023b (2019). [DOI] [PubMed] [Google Scholar]
- 185.Huang D, Patel K, Perez-Garrido S, Marshall JF & Palma M DNA Origami Nanoarrays for Multivalent Investigations of Cancer Cell Spreading with Nanoscale Spatial Resolution and Single-Molecule Control. ACS Nano 13, 728–736, doi: 10.1021/acsnano.8b08010 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Karna D et al. Chemo-Mechanical Modulation of Cell Motions Using DNA Nanosprings. Bioconjug Chem 32, 311–317, doi: 10.1021/acs.bioconjchem.0c00674 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang Y, Baars I, Fordos F & Hogberg B Clustering of Death Receptor for Apoptosis Using Nanoscale Patterns of Peptides. ACS Nano 15, 9614–9626, doi: 10.1021/acsnano.0c10104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bian X, Zhang Z, Xiong Q, De Camilli P & Lin C A programmable DNA-origami platform for studying lipid transfer between bilayers. Nat Chem Biol 15, 830–837, doi: 10.1038/s41589-019-0325-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dong Y et al. Folding DNA into a Lipid-Conjugated Nanobarrel for Controlled Reconstitution of Membrane Proteins. Angew Chem Int Ed Engl 57, 2072–2076, doi: 10.1002/anie.201710147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang Y et al. Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat Chem 8, 476–483, doi: 10.1038/nchem.2472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang Z & Chapman ER Programmable Nanodisc Patterning by DNA Origami. Nano Lett 20, 6032–6037, doi: 10.1021/acs.nanolett.0c02048 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang Z, Yang Y, Pincet F, Llaguno MC & Lin C Placing and shaping liposomes with reconfigurable DNA nanocages. Nat Chem 9, 653–659, doi: 10.1038/nchem.2802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao Z et al. DNA-Corralled Nanodiscs for the Structural and Functional Characterization of Membrane Proteins and Viral Entry. J Am Chem Soc 140, 10639–10643, doi: 10.1021/jacs.8b04638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gopfrich K et al. Large-Conductance Transmembrane Porin Made from DNA Origami. ACS Nano 10, 8207–8214, doi: 10.1021/acsnano.6b03759 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Du Y et al. DNA-Nanostructure-Gold-Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Adv Mater 28, 10000–10007, doi: 10.1002/adma.201601710 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Jiang D et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat Biomed Eng 2, 865–877, doi: 10.1038/s41551-018-0317-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang Q et al. A Self-Assembled DNA Origami-Gold Nanorod Complex for Cancer Theranostics. Small 11, 5134–5141, doi: 10.1002/smll.201501266 (2015). [DOI] [PubMed] [Google Scholar]
- 198.Kaur V, Tanwar S, Kaur G & Sen T DNA-Origami-Based Assembly of Au@Ag Nanostar Dimer Nanoantennas for Label-Free Sensing of Pyocyanin. Chemphyschem 22, 160–167, doi: 10.1002/cphc.202000805 (2021). [DOI] [PubMed] [Google Scholar]
- 199.Meyer TA, Zhang C, Bao G & Ke Y Programmable Assembly of Iron Oxide Nanoparticles Using DNA Origami. Nano Lett 20, 2799–2805, doi: 10.1021/acs.nanolett.0c00484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Song L et al. DNA origami/gold nanorod hybrid nanostructures for the circumvention of drug resistance. Nanoscale 9, 7750–7754, doi: 10.1039/c7nr02222k (2017). [DOI] [PubMed] [Google Scholar]
- 201.Takenaka T et al. Photoresponsive DNA nanocapsule having an open/close system for capture and release of nanomaterials. Chemistry 20, 14951–14954, doi: 10.1002/chem.201404757 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Agarwal NP, Matthies M, Gur FN, Osada K & Schmidt TL Block Copolymer Micellization as a Protection Strategy for DNA Origami. Angew Chem Int Ed Engl 56, 5460–5464, doi: 10.1002/anie.201608873 (2017). [DOI] [PubMed] [Google Scholar]
- 203.Liu J et al. A DNA-Based Nanocarrier for Efficient Gene Delivery and Combined Cancer Therapy. Nano Lett 18, 3328–3334, doi: 10.1021/acs.nanolett.7b04812 (2018). [DOI] [PubMed] [Google Scholar]
- 204.Kim Y & Yin P Enhancing Biocompatible Stability of DNA Nanostructures Using Dendritic Oligonucleotides and Brick Motifs. Angew Chem Int Ed Engl 59, 700–703, doi: 10.1002/anie.201911664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nakata E et al. Zinc-finger proteins for site-specific protein positioning on DNA-origami structures. Angew Chem Int Ed Engl 51, 2421–2424, doi: 10.1002/anie.201108199 (2012). [DOI] [PubMed] [Google Scholar]
- 206.Sagredo S et al. Orthogonal Protein Assembly on DNA Nanostructures Using Relaxases. Angew Chem Int Ed Engl 55, 4348–4352, doi: 10.1002/anie.201510313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kurokawa T et al. DNA Origami Scaffolds as Templates for Functional Tetrameric Kir3 K(+) Channels. Angew Chem Int Ed Engl 57, 2586–2591, doi: 10.1002/anie.201709982 (2018). [DOI] [PubMed] [Google Scholar]
- 208.Takusagawa M et al. HBD1 protein with a tandem repeat of two HMG-box domains is a DNA clip to organize chloroplast nucleoids in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2021053118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aksel T, Yu Z, Cheng Y & Douglas SM Molecular goniometers for single-particle cryo-electron microscopy of DNA-binding proteins. Nat Biotechnol 39, 378–386, doi: 10.1038/s41587-020-0716-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kuzuya A et al. Precisely programmed and robust 2D streptavidin nanoarrays by using periodical nanometer-scale wells embedded in DNA origami assembly. Chembiochem 10, 1811–1815, doi: 10.1002/cbic.200900229 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Kuzyk A, Laitinen KT & Torma P DNA origami as a nanoscale template for protein assembly. Nanotechnology 20, 235305, doi: 10.1088/0957-4484/20/23/235305 (2009). [DOI] [PubMed] [Google Scholar]
- 212.Le JV et al. Probing Nucleosome Stability with a DNA Origami Nanocaliper. ACS Nano 10, 7073–7084, doi: 10.1021/acsnano.6b03218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Linko V, Eerikainen M & Kostiainen MA A modular DNA origami-based enzyme cascade nanoreactor. Chem Commun (Camb) 51, 5351–5354, doi: 10.1039/c4cc08472a (2015). [DOI] [PubMed] [Google Scholar]
- 214.Mallik L et al. Electron Microscopic Visualization of Protein Assemblies on Flattened DNA Origami. ACS Nano 9, 7133–7141, doi: 10.1021/acsnano.5b01841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Numajiri K, Kimura M, Kuzuya A & Komiyama M Stepwise and reversible nanopatterning of proteins on a DNA origami scaffold. Chem Commun (Camb) 46, 5127–5129, doi: 10.1039/c0cc00044b (2010). [DOI] [PubMed] [Google Scholar]
- 216.Ora A et al. Cellular delivery of enzyme-loaded DNA origami. Chem Commun (Camb) 52, 14161–14164, doi: 10.1039/c6cc08197e (2016). [DOI] [PubMed] [Google Scholar]
- 217.Timm C & Niemeyer CM Assembly and purification of enzyme-functionalized DNA origami structures. Angew Chem Int Ed Engl 54, 6745–6750, doi: 10.1002/anie.201500175 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Yamazaki T, Heddle JG, Kuzuya A & Komiyama M Orthogonal enzyme arrays on a DNA origami scaffold bearing size-tunable wells. Nanoscale 6, 9122–9126, doi: 10.1039/c4nr01598c (2014). [DOI] [PubMed] [Google Scholar]
- 219.Yan J et al. Novel rolling circle amplification and DNA origami-based DNA belt-involved signal amplification assay for highly sensitive detection of prostate-specific antigen (PSA). ACS Appl Mater Interfaces 6, 20372–20377, doi: 10.1021/am505913d (2014). [DOI] [PubMed] [Google Scholar]
- 220.Pedersen RO, Loboa EG & LaBean TH Sensitization of transforming growth factor-beta signaling by multiple peptides patterned on DNA nanostructures. Biomacromolecules 14, 4157–4160, doi: 10.1021/bm4011722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang Q et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 8, 6633–6643, doi: 10.1021/nn502058j (2014). [DOI] [PubMed] [Google Scholar]
- 222.Breitz HB et al. Clinical optimization of pretargeted radioimmunotherapy with antibody-streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med 41, 131–140 (2000). [PubMed] [Google Scholar]
- 223.Meyer DL et al. Reduced antibody response to streptavidin through site-directed mutagenesis. Protein Sci 10, 491–503, doi: 10.1110/ps.19901 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mao X et al. Directing curli polymerization with DNA origami nucleators. Nat Commun 10, 1395, doi: 10.1038/s41467-019-09369-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shen W, Zhong H, Neff D & Norton ML NTA directed protein nanopatterning on DNA Origami nanoconstructs. J Am Chem Soc 131, 6660–6661, doi: 10.1021/ja901407j (2009). [DOI] [PubMed] [Google Scholar]
- 226.Brglez J, Nikolov P, Angelin A & Niemeyer CM Designed Intercalators for Modification of DNA Origami Surface Properties. Chemistry 21, 9440–9446, doi: 10.1002/chem.201500086 (2015). [DOI] [PubMed] [Google Scholar]
- 227.Halley PD et al. Daunorubicin-Loaded DNA Origami Nanostructures Circumvent Drug-Resistance Mechanisms in a Leukemia Model. Small 12, 308–320, doi: 10.1002/smll.201502118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jiang Q et al. DNA origami as a carrier for circumvention of drug resistance. J Am Chem Soc 134, 13396–13403, doi: 10.1021/ja304263n (2012). [DOI] [PubMed] [Google Scholar]
- 229.Miller HL et al. Biophysical characterisation of DNA origami nanostructures reveals inaccessibility to intercalation binding sites. Nanotechnology 31, 235605, doi: 10.1088/1361-6528/ab7a2b (2020). [DOI] [PubMed] [Google Scholar]
- 230.Palazzolo S et al. An Effective Multi-Stage Liposomal DNA Origami Nanosystem for In Vivo Cancer Therapy. Cancers (Basel) 11, doi: 10.3390/cancers11121997 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Palazzolo S et al. Proof-of-Concept Multistage Biomimetic Liposomal DNA Origami Nanosystem for the Remote Loading of Doxorubicin. ACS Med Chem Lett 10, 517–521, doi: 10.1021/acsmedchemlett.8b00557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pan QS et al. Aptamer-Functionalized DNA Origami for Targeted Codelivery of Antisense Oligonucleotides and Doxorubicin to Enhance Therapy in Drug-Resistant Cancer Cells. Acs Appl Mater Inter 12, 400–409, doi: 10.1021/acsami.9b20707 (2020). [DOI] [PubMed] [Google Scholar]
- 233.Zeng Y et al. Time-lapse live cell imaging to monitor doxorubicin release from DNA origami nanostructures. J Mater Chem B 6, 1605–1612, doi: 10.1039/C7TB03223D (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhao YX et al. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano 6, 8684–8691, doi: 10.1021/nn3022662 (2012). [DOI] [PubMed] [Google Scholar]
- 235.Zhuang X et al. A Photosensitizer-Loaded DNA Origami Nanosystem for Photodynamic Therapy. ACS Nano 10, 3486–3495, doi: 10.1021/acsnano.5b07671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ijas H et al. Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release. Nucleic Acids Res 49, 3048–3062, doi: 10.1093/nar/gkab097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mikkila J et al. Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett 14, 2196–2200, doi: 10.1021/nl500677j (2014). [DOI] [PubMed] [Google Scholar]
- 238.Auvinen H et al. Protein Coating of DNA Nanostructures for Enhanced Stability and Immunocompatibility. Adv Healthc Mater 6, doi: 10.1002/adhm.201700692 (2017). [DOI] [PubMed] [Google Scholar]
- 239.Hernandez-Garcia A et al. Precise Coating of a Wide Range of DNA Templates by a Protein Polymer with a DNA Binding Domain. ACS Nano 11, 144–152, doi: 10.1021/acsnano.6b05938 (2017). [DOI] [PubMed] [Google Scholar]
- 240.Kopatz I et al. Packaging of DNA origami in viral capsids. Nanoscale 11, 10160–10166, doi: 10.1039/c8nr10113b (2019). [DOI] [PubMed] [Google Scholar]
- 241.Xu X et al. Cationic Albumin Encapsulated DNA Origami for Enhanced Cellular Transfection and Stability. Materials (Basel) 12, doi: 10.3390/ma12060949 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang Y et al. A nanoscale DNA force spectrometer capable of applying tension and compression on biomolecules. Nucleic Acids Res 49, 8987–8999, doi: 10.1093/nar/gkab656 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shen X et al. Visualization of the intracellular location and stability of DNA origami with a label-free fluorescent probe. Chem Commun (Camb) 48, 11301–11303, doi: 10.1039/c2cc36185j (2012). [DOI] [PubMed] [Google Scholar]
- 244.Kiviaho JK et al. Cationic polymers for DNA origami coating - examining their binding efficiency and tuning the enzymatic reaction rates. Nanoscale 8, 11674–11680, doi: 10.1039/c5nr08355a (2016). [DOI] [PubMed] [Google Scholar]
- 245.Ponnuswamy N et al. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat Commun 8, 15654, doi: 10.1038/ncomms15654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ahmadi Y, De Llano E & Barisic I (Poly)cation-induced protection of conventional and wireframe DNA origami nanostructures. Nanoscale 10, 7494–7504, doi: 10.1039/c7nr09461b (2018). [DOI] [PubMed] [Google Scholar]
- 247.Ahmadi Y & Barisic I Gene-therapy Inspired Polycation Coating for Protection of DNA Origami Nanostructures. J Vis Exp, doi: 10.3791/58771 (2019). [DOI] [PubMed] [Google Scholar]
- 248.Wang ST et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc Natl Acad Sci U S A 117, 6339–6348, doi: 10.1073/pnas.1919749117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Anastassacos FM, Zhao Z, Zeng Y & Shih WM Glutaraldehyde Cross-Linking of Oligolysines Coating DNA Origami Greatly Reduces Susceptibility to Nuclease Degradation. J Am Chem Soc 142, 3311–3315, doi: 10.1021/jacs.9b11698 (2020). [DOI] [PubMed] [Google Scholar]
- 250.Estrich NA, Hernandez-Garcia A, de Vries R & LaBean TH Engineered Diblock Polypeptides Improve DNA and Gold Solubility during Molecular Assembly. ACS Nano 11, 831–842, doi: 10.1021/acsnano.6b07291 (2017). [DOI] [PubMed] [Google Scholar]
- 251.Julin S, Nonappa Shen B., Linko V & Kostiainen MA DNA-Origami-Templated Growth of Multilamellar Lipid Assemblies. Angew Chem Int Ed Engl 60, 827–833, doi: 10.1002/anie.202006044 (2021). [DOI] [PubMed] [Google Scholar]
- 252.Shaukat A et al. Phthalocyanine-DNA origami complexes with enhanced stability and optical properties. Chem Commun (Camb) 56, 7341–7344, doi: 10.1039/d0cc01916j (2020). [DOI] [PubMed] [Google Scholar]
- 253.Sacca B et al. Orthogonal protein decoration of DNA origami. Angew Chem Int Ed Engl 49, 9378–9383, doi: 10.1002/anie.201005931 (2010). [DOI] [PubMed] [Google Scholar]
- 254.Masubuchi T et al. Construction of integrated gene logic-chip. Nat Nanotechnol 13, 933–940, doi: 10.1038/s41565-018-0202-3 (2018). [DOI] [PubMed] [Google Scholar]
- 255.Nguyen TM, Nakata E, Saimura M, Dinh H & Morii T Design of Modular Protein Tags for Orthogonal Covalent Bond Formation at Specific DNA Sequences. J Am Chem Soc 139, 8487–8496, doi: 10.1021/jacs.7b01640 (2017). [DOI] [PubMed] [Google Scholar]
- 256.Angelin A, Kassel O, Rastegar S, Strahle U & Niemeyer CM Protein-Functionalized DNA Nanostructures as Tools to Control Transcription in Zebrafish Embryos. ChemistryOpen 6, 33–39, doi: 10.1002/open.201600153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nakata E, Dinh H, Ngo TA, Saimura M & Morii T A modular zinc finger adaptor accelerates the covalent linkage of proteins at specific locations on DNA nanoscaffolds. Chem Commun (Camb) 51, 1016–1019, doi: 10.1039/c4cc08167f (2015). [DOI] [PubMed] [Google Scholar]
- 258.Burgahn T, Garrecht R, Rabe KS & Niemeyer CM Solid-Phase Synthesis and Purification of Protein-DNA Origami Nanostructures. Chemistry 25, 3483–3488, doi: 10.1002/chem.201805506 (2019). [DOI] [PubMed] [Google Scholar]
- 259.Kossmann KJ et al. A Rationally Designed Connector for Assembly of Protein-Functionalized DNA Nanostructures. Chembiochem 17, 1102–1106, doi: 10.1002/cbic.201600039 (2016). [DOI] [PubMed] [Google Scholar]
- 260.Zhang Z et al. Tuning the Reactivity of a Substrate for SNAP-Tag Expands Its Application for Recognition-Driven DNA-Protein Conjugation. Chemistry, doi: 10.1002/chem.202103304 (2021). [DOI] [PubMed] [Google Scholar]
- 261.Kroll S, Rabe KS & Niemeyer CM An Orthogonal Covalent Connector System for the Efficient Assembly of Enzyme Cascades on DNA Nanostructures. Small, e2105095, doi: 10.1002/smll.202105095 (2021). [DOI] [PubMed] [Google Scholar]
- 262.Stephanopoulos N & Francis MB Choosing an effective protein bioconjugation strategy. Nat Chem Biol 7, 876–884, doi: 10.1038/nchembio.720 (2011). [DOI] [PubMed] [Google Scholar]
- 263.Ouyang X et al. Docking of Antibodies into the Cavities of DNA Origami Structures. Angew Chem Int Ed Engl 56, 14423–14427, doi: 10.1002/anie.201706765 (2017). [DOI] [PubMed] [Google Scholar]
- 264.Voigt NV et al. Single-molecule chemical reactions on DNA origami. Nat Nanotechnol 5, 200–203, doi: 10.1038/nnano.2010.5 (2010). [DOI] [PubMed] [Google Scholar]
- 265.Kolb HC, Finn MG & Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl 40, 2004–2021, doi: (2001). [DOI] [PubMed] [Google Scholar]
- 266.Raniolo S et al. Cellular uptake of covalent and non-covalent DNA nanostructures with different sizes and geometries. Nanoscale 11, 10808–10818, doi: 10.1039/c9nr02006c (2019). [DOI] [PubMed] [Google Scholar]
- 267.Dommerholt J, Rutjes F & van Delft FL Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Top Curr Chem (Cham) 374, 16, doi: 10.1007/s41061-016-0016-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.McKay CS & Finn MG Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol 21, 1075–1101, doi: 10.1016/j.chembiol.2014.09.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lin Z, Xiong Y, Xiang S & Gang O Controllable Covalent-Bound Nanoarchitectures from DNA Frames. J Am Chem Soc 141, 6797–6801, doi: 10.1021/jacs.9b01510 (2019). [DOI] [PubMed] [Google Scholar]
- 270.Heck C et al. Label as you fold: methyltransferase-assisted functionalization of DNA nanostructures. Nanoscale 12, 20287–20291, doi: 10.1039/d0nr03694c (2020). [DOI] [PubMed] [Google Scholar]
- 271.Knappe GA, Wamhoff EC, Read BJ, Irvine DJ & Bathe M In Situ Covalent Functionalization of DNA Origami Virus-like Particles. ACS Nano 15, 14316–14322, doi: 10.1021/acsnano.1c03158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Oliveira BL, Guo Z & Bernardes GJL Inverse electron demand Diels-Alder reactions in chemical biology. Chem Soc Rev 46, 4895–4950, doi: 10.1039/c7cs00184c (2017). [DOI] [PubMed] [Google Scholar]
- 273.Zhang JX et al. Predicting DNA hybridization kinetics from sequence. Nat Chem 10, 91–98, doi: 10.1038/nchem.2877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Srisa-Art M, Dyson EC, deMello AJ & Edel JB Monitoring of real-time streptavidin-biotin binding kinetics using droplet microfluidics. Anal Chem 80, 7063–7067, doi: 10.1021/ac801199k (2008). [DOI] [PubMed] [Google Scholar]
- 275.Kanekiyo M et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol 20, 362–372, doi: 10.1038/s41590-018-0305-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cohen AA et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 371, 735–741, doi: 10.1126/science.abf6840 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wagenbauer KF et al. How We Make DNA Origami. Chembiochem 18, 1873–1885, doi: 10.1002/cbic.201700377 (2017). [DOI] [PubMed] [Google Scholar]
- 278.Cervantes-Salguero K, Freeley M, Chavez JL & Palma M Single-molecule DNA origami aptasensors for real-time biomarker detection. J Mater Chem B 8, 6352–6356, doi: 10.1039/d0tb01291b (2020). [DOI] [PubMed] [Google Scholar]
- 279.Lin C, Perrault SD, Kwak M, Graf F & Shih WM Purification of DNA-origami nanostructures by rate-zonal centrifugation. Nucleic Acids Res 41, e40, doi: 10.1093/nar/gks1070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stahl E, Martin TG, Praetorius F & Dietz H Facile and scalable preparation of pure and dense DNA origami solutions. Angew Chem Int Ed Engl 53, 12735–12740, doi: 10.1002/anie.201405991 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fahrner RL et al. Industrial purification of pharmaceutical antibodies: development, operation, and validation of chromatography processes. Biotechnol Genet Eng Rev 18, 301–327, doi: 10.1080/02648725.2001.10648017 (2001). [DOI] [PubMed] [Google Scholar]
- 282.Johnston A & Adcock W The use of chromatography to manufacture purer and safer plasma products. Biotechnol Genet Eng Rev 17, 37–70, doi: 10.1080/02648725.2000.10647987 (2000). [DOI] [PubMed] [Google Scholar]
- 283.Mathur D & Medintz IL Analyzing DNA Nanotechnology: A Call to Arms For The Analytical Chemistry Community. Anal Chem 89, 2646–2663, doi: 10.1021/acs.analchem.6b04033 (2017). [DOI] [PubMed] [Google Scholar]
- 284.Mehnert W & Mader K Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47, 165–196, doi: 10.1016/s0169-409x(01)00105-3 (2001). [DOI] [PubMed] [Google Scholar]
- 285.Kretschy N, Sack M & Somoza MM Sequence-Dependent Fluorescence of Cy3- and Cy5-Labeled Double-Stranded DNA. Bioconjug Chem 27, 840–848, doi: 10.1021/acs.bioconjchem.6b00053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Funke JJ & Dietz H Placing molecules with Bohr radius resolution using DNA origami. Nat Nanotechnol 11, 47–52, doi: 10.1038/nnano.2015.240 (2016). [DOI] [PubMed] [Google Scholar]
- 287.Strauss MT, Schueder F, Haas D, Nickels PC & Jungmann R Quantifying absolute addressability in DNA origami with molecular resolution. Nat Commun 9, 1600, doi: 10.1038/s41467-018-04031-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bertosin E et al. Cryo-Electron Microscopy and Mass Analysis of Oligolysine-Coated DNA Nanostructures. ACS Nano 15, 9391–9403, doi: 10.1021/acsnano.0c10137 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.van Dyck JF et al. Sizing up DNA nanostructure assembly with native mass spectrometry and ion mobility. Nat Commun 13, 3610, doi: 10.1038/s41467-022-31029-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jain SS & Tullius TD Footprinting protein-DNA complexes using the hydroxyl radical. Nat Protoc 3, 1092–1100, doi: 10.1038/nprot.2008.72 (2008). [DOI] [PubMed] [Google Scholar]
- 291.Weeks KM Advances in RNA structure analysis by chemical probing. Curr Opin Struct Biol 20, 295–304, doi: 10.1016/j.sbi.2010.04.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zubradt M et al. DMS-MaPseq for genome-wide or targeted RNA structure probing in vivo. Nat Methods 14, 75–82, doi: 10.1038/nmeth.4057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.