Fig. 7.
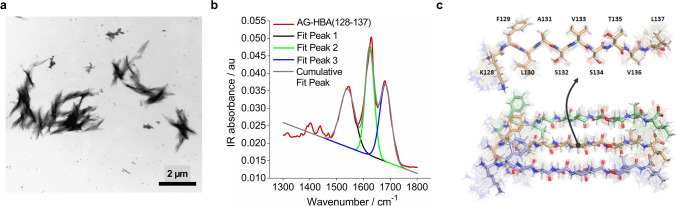
Biophysical characteristics of HBA(128–137). a TEM images of AG-HBA(128–137). Fibrils were negatively stained with 2% uranyl acetate in water on copper grids, and imaged with a Jeol TEM 1400. b ATR FT-IR spectra of AG-HBA(128–137). Shown is the peak deconvolution of the spectra using three components with peak maxima at 1,539 cm−1 (fit 1), 1627 cm−1 (fit 2), and 1674 cm−1 (fit 3) using gaussian shape fits. The peaks can be assigned to the amide II β-sheet, amide I β-sheet and β-turn structures, respectively. The integral ratio between fit 2 and fit 3 was used to quantify the β-sheet amount. c NMR-based structure of the HBA(128–137) 10-mer peptide as calculated in the context of a symmetrical in-register parallel beta-sheet (bottom). The residues are labeled on the monomeric unit (top, about 90 degrees rotated). The lowest energy structure is highlighted and the full ensemble of the best 20 structures is shown faded in the background. The Fig. was generated using PyMOL