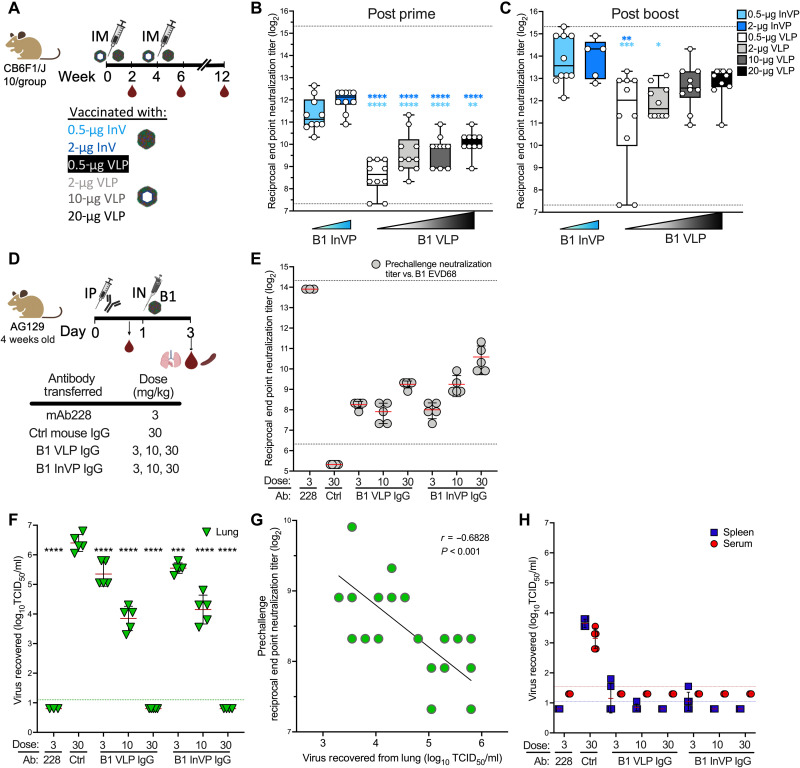
Fig. 2. B1 VLP and B1 InVP elicit potent neutralizing antibody that blocks EV-D68 in cell culture and abrogates lung replication and dissemination in vivo.
(A) Vaccination schema. Serum was obtained on weeks 2, 6, and 12. IM, intramuscularly. (B and C) Neutralization of EV-D68 US/MO/2014-18947 at week 2 (B) or week 6 (C). Dotted lines indicate limits of detection; error bars indicate ±SD. Ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare neutralizing titers at each time point. Light blue and dark blue asterisks indicate comparison of the VLP to 0.5 μg of InVP and 2.0 μg of InVP, respectively. (D) Antibody transfer study schema. IgG from VLP- or InVP-vaccinated mice at week 12 were purified and injected intraperitoneally (IP) into AG129 mice at the indicated concentration (n = 3 to 5 mice per group). Serum was taken to confirm the transfer of neutralizing antibodies. IN, intranasally. (E) B1 subclade EV-D68 US/MO/2014-18947 end point neutralization titers before virus challenge. Dotted lines indicate limits of detection; error bars indicate ±SD. Mice were challenged intranasally with 104.4 median tissue culture infectious dose (TCID50) B1 subclade EV-D68 Mp40. Two days after challenge, lungs, spleen, and serum were taken for assessment of viral load. (F) Viral lung titers demonstrate an antibody dose-dependent decrease in virus replication in the lung. Ordinary one-way ANOVA with Dunnett’s multiple comparisons test was used to determine significance relative to the naïve IgG control (30 mg/kg). The dotted line indicates the lower limit of detection in the assay; error bars indicate ±SD. (G) Correlation of lung viral load and serum end point neutralization titer in pre-challenge serum samples. (H) Viral titers, measured by TCID50 assay, demonstrate that IgG from vaccinated mice inhibit mouse-adapted EV-D68 dissemination to the spleen and serum. Blue and red dotted lines indicate the limit of virus detection in spleen and serum, respectively; error bars indicate ±SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.