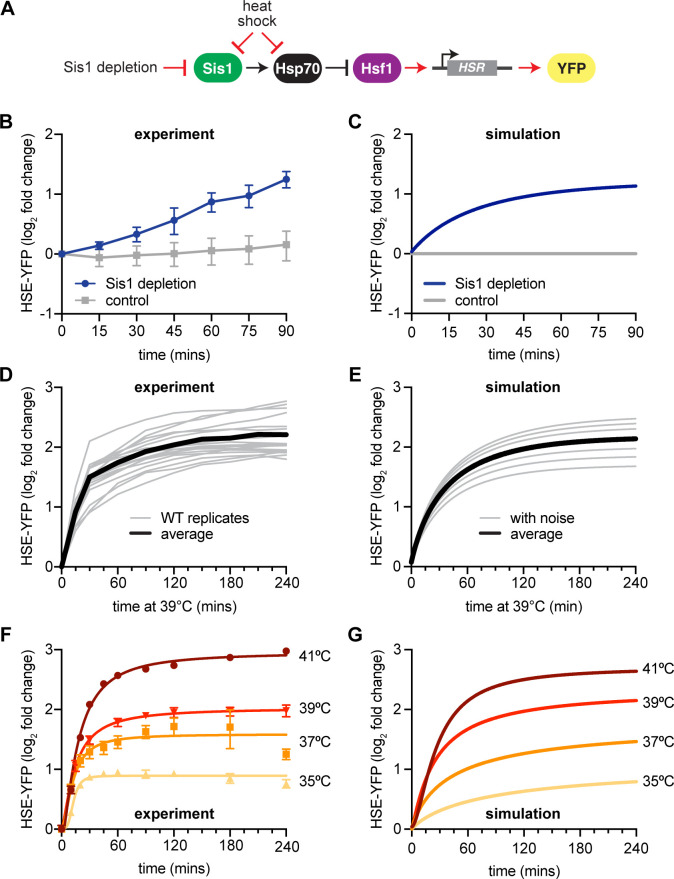
Figure 2. New heat shock response (HSR) model recapitulates experimental results.
(A) Model of Sis1 effect. In non-heat shock, Sis1 promotes Hsp70 binding to Hsf1, repressing transcriptional activation of the HSR. Heat shock results in the titration of Hsp70 and Sis1, leaving Hsf1 free to induce the HSR and the ectopic reporter of Hsf1 activity, HSE-YFP (red arrows). (B) HSE-YFP levels after nuclear Sis1 depletion (blue) at 30°C, measured by flow cytometry. Sis1 was anchored away to cytosolic ribosomes using 10 µg/ml rapamycin and the time course was started immediately. (C) Simulation of HSE-YFP reporter after Sis1 depletion. (D) HSE-YFP expression in wild-type cells across 18 biological replicates (gray). Average in black. (E) Simulation of variation in the wild-type HSR due to metabolic differences leading to changes in basal translation rate. (F) HSE-YFP heat shock time courses at a range of heat shock temperatures between 35 and 41°C. (G) Simulation of heat shock time courses at different induction temperatures. Heat shock at higher temperatures was simulated by a proportional decrease in protein folding rate.