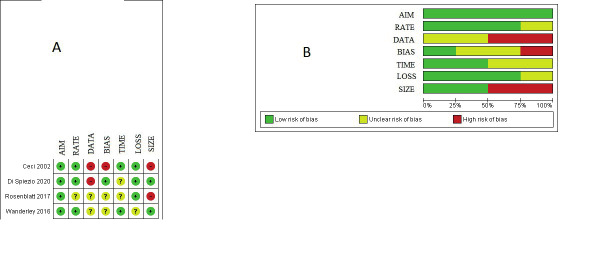
Figure 2.
Assessment of risk of bias. Aim, clearly stated aim; Rate, inclusion of consecutive patients and response rate; Data, prospective collection of data; Bias, unbiased assessment of study end points; Time, follow-up time appropriate; Loss, loss to follow-up; Size, calculation of the sample size. (A) Summary of risk of bias for each study. Plus sign, low risk of bias; minus sign, high risk of bias; question mark, unclear risk of bias. (B) Risk of bias graph about each risk of bias item presented as percentages across all included studies.