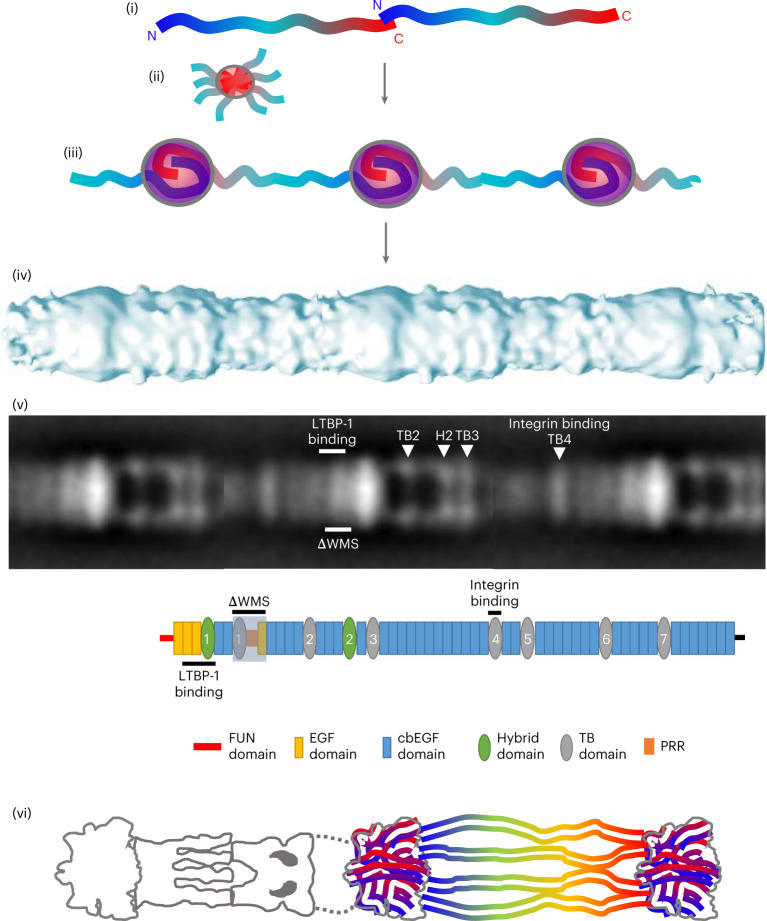
Fig. 7. Fibrillin assembly and organization.
(i) Published data support an initial head-to-tail assembly of fibrillin molecules mediated via an interaction between domains FUN–EGF1 and cbEGF41–43 (refs. 14,49). Subsequent assembly steps result in a mature beaded microfibril with N- and C-terminal antibody epitopes either side of the bead region, pseudo eightfold symmetry and 57 nm periodicity. (ii) The C-terminal region of fibrillin is likely to facilitate assembly as the C-terminal half of fibrillin-1 can independently multimerize into bead-like structures16. (iii) The bead has a mass of ~1.1 MDa, as determined by STEM mass mapping and the cryo-EM density map, so if eight pairs of fibrillin molecules overlap in a head-to-tail manner within the bead, the mass would relate to >20 fibrillin domains present for each overlapping pair of molecules. This supports more extensive secondary interactions15,50 and packing of terminal regions within the bead. (iv) The mature microfibril has a 3D cylindrical structure with pronounced beads on a string when viewed in projection. (v) Regions mapped in this study are shown on a fibrillin microfibril negative stain class average, with the same regions shown below on a schematic of the fibrillin molecule. (vi) Together our data show that the bead is dense and interwoven, supporting condensation of N- and C-terminal regions within and the location of other regions of the fibrillin molecule within the arm, interbead and shoulder regions.