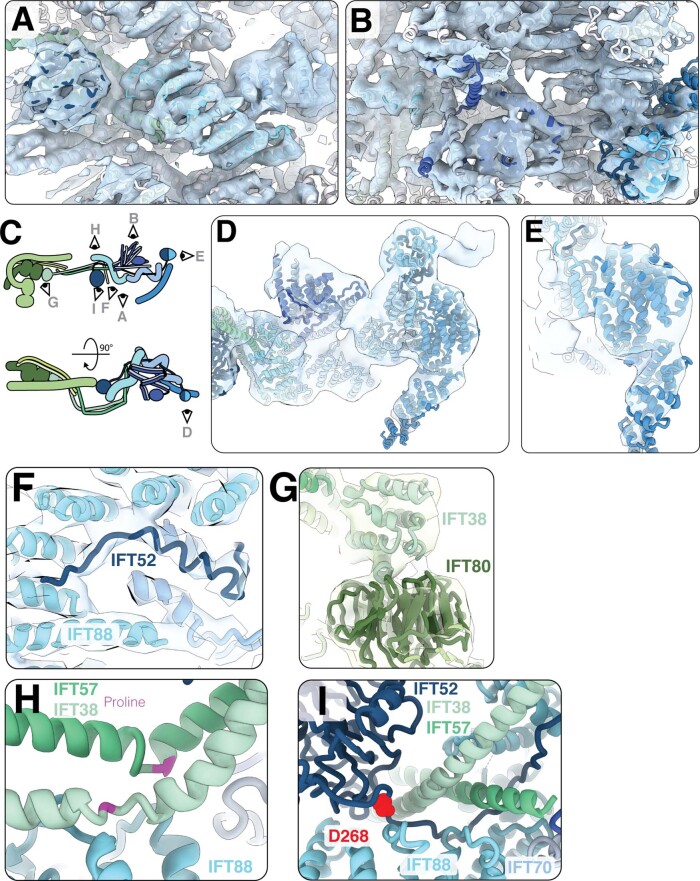
Extended Data Fig. 6. Building a model of IFT-B1.
a, A view of the IFT-B1 model docked into its density from the bottom (see E). b, A view of the IFT-B1 model docked into its density from the top (see E). c, Cartoon representation of IFT-B showing the views in A-D. d, A side view of the ‘tail’ of IFT-B1 docked into the masked tail refinement (Extended data 2A) map lowpass filtered to 18 Å. The region containing IFT56 was more flexible in the high-resolution average shown in A/B, but is more clearly resolved here. e, A close up view of IFT56 in the masked tail refinement map, showing that the twist in the TPR helix is visible. f, Density for the central unstructured domain of IFT52 (dark blue) is visible in the central pore of IFT88 (cyan), showing that the Alphafold2 prediction agrees with our experimental data. g, The N-terminal CH domain of IFT37 (light green) docks to the exterior face of the first WD domain of IFT80 (dark green) in IFT-B2. h, A proline residue (magenta) creates a kink in each of the IFT57/38 (dark/light green) helices near the contact to the first IFT88. i, The position of D268 in IFT52 highlighted in red, at the interface between IFT-B1 and IFT-B2. D268 in C. reinhardtii corresponds to the D259H mutation in humans22.