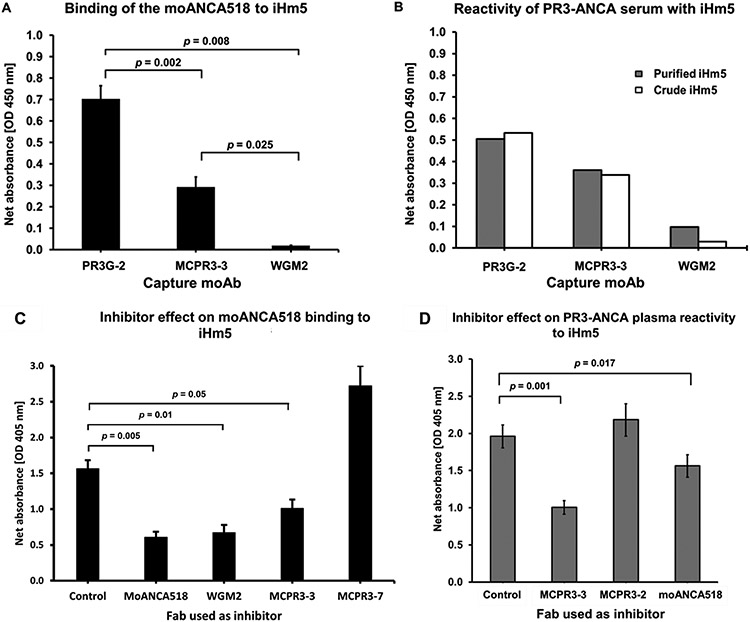
Fig. 4 – Identification of Epitope 3 of iHm5 as a target of moANCA518 and PR3-ANCA.
A. Inhibition of moANCA518 binding to iHm5 using different moAbs to capture antigen iHm5. Binding of moANCA518 to iHm5 was impaired significantly when the moAbs MCPR3-3 and WGM2 (recognizing Epitope 3) were used to capture the antigen, compared to PR3G-2 which binds (recognizing Epitope 1). This previously shown data (32) is included again here to illustrate similarities with the PR3-ANCA serum results (panel B).
B. Serum PR3-ANCA from the same patient and study visit from which moANCA518 was derived from plasmablasts yields similar differential reactivity of PR3-ANCA with iHm5 captured by the different moAbs Results for purified iHm5 antigen [black bars] or crude iHm5 contained in media supernatant from cells expressing the recombinant antigen [white bars] were similar.
C. Fabs from epitope-specific moAbs as competitors of binding of moANCA518 to iHm5 using the anchor ELISA. Compared to control, binding of moANCA518 to iHm5 was reduced significantly by moANCA518 Fab (p=0.005) and Fabs MCPR3-3 (p=0.005) and WGM2 (p=0.01) but not Fabs from MCPR3-7 (recognizing Epitope 5).
D. Binding of PR3-ANCA to iHm5 in the anchor ELISA was similarly inhibited by Fabs recognizing Epitope 3 (MCPR3-3 and moANCA518). Comparison in panel C and D are with control mouse IgG1 Fab.