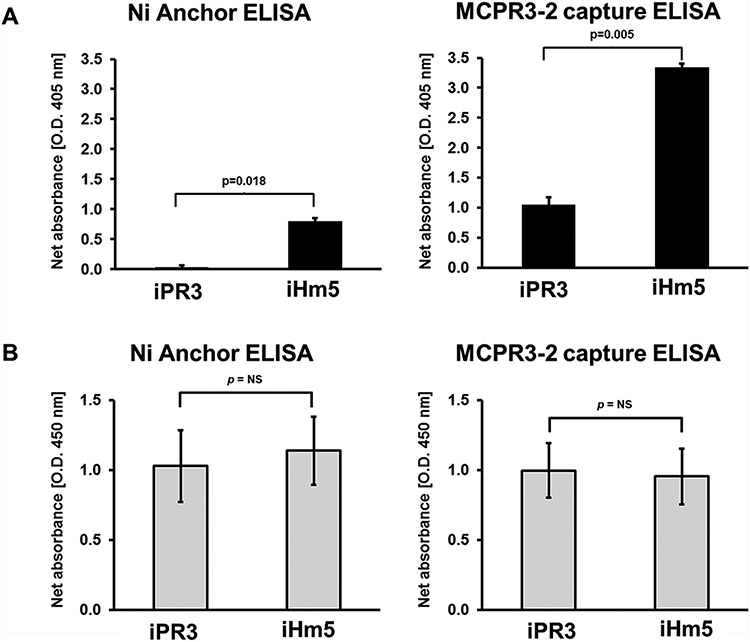
Fig. 5 – Binding to monoclonal antibodies facilitates iPR3 recognition by the moANCA518.
A. Detection of the binding of moANCA518 to iHm5 and iPR3 using anchor ELISA and MCPR3-2 capture ELISA. Binding of the moANCA518 to iHm5 was detected by anchor ELISA, whereas no binding to iPR3 could be detected (p=0.018). In contrast, on the MCPR3-2 capture ELISA, the moANCA518 bound to both constructs, iHm5 and iPR3, but the signal of the binding to iHm5 was higher when compared with the signal of the binding to iPR3 (p=0.005).
B. Control experiments documenting comparable coating of iPR3 and iHm5, on the anchor ELISA, and comparable coating of MCRP3-2 bound to iPR3 and iHm5, on the MCPR3-2 capture ELISA (p=ns) detected by polyclonal rabbit anti-PR3 antibody. (Abbreviations: ELISA - enzyme-linked immunosorbent assay; PR3 - proteinase 3)