Figure 1. Key figure. Molecular analogy in the microenvironment of metastasis and regenerating benign tissue.
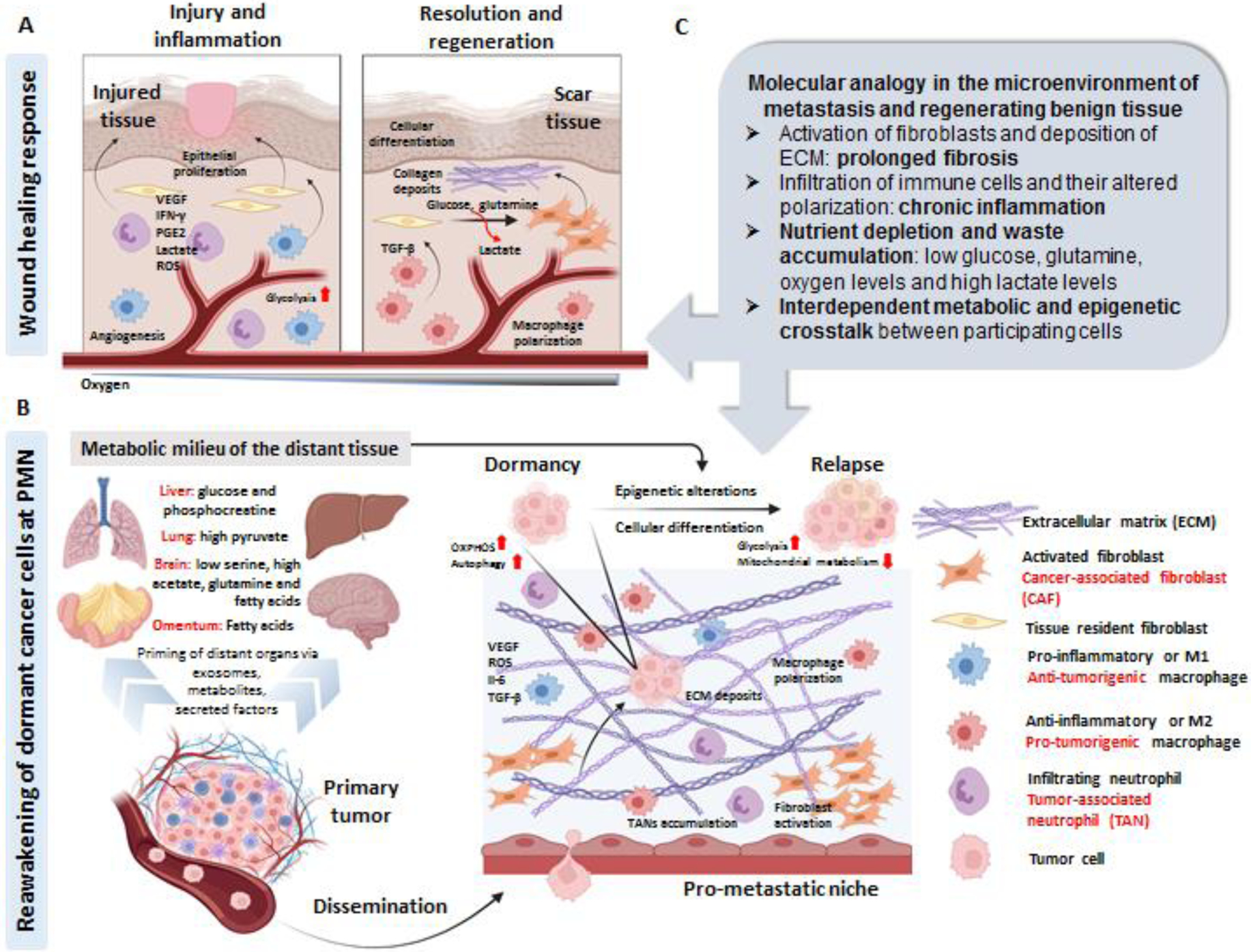
A. During the initial phase of the physiologic wound healing response, there is a cascade of pro-inflammatory events including neutrophil and macrophage infiltration/activation, angiogenesis (or neo-vascularization: generation of new blood vessels) and epithelial proliferation. The accumulated lactate in the microenvironment (due to the high glycolysis rates of the pro-inflammatory cells and poor oxygenation in the wound) polarizes the macrophages to an anti-inflammatory phenotype, which in turn leads to activation of tissue-resident fibroblasts. Highly proliferative, activated (myo)-fibroblasts utilize glucose and glutamine for their energy consumption, leading to more lactate release (that further promotes angiogenesis), with a concurrently ECM deposition to form scar tissue. B. Circulating exosomes, metabolites and secreted factors from the primary tumor primes different organs of the body from a very early stage of cancer progression leading to the formation of prometastatic niche (PMN), which is comprised of activated fibroblasts, ECM deposits, and an inflamed parenchyma (caused by an intricate interplay between the pro and anti-tumorigenic neutrophils, macrophages and T-cells). As the cancer progresses, disseminated tumor cells are passively trapped or actively seeded in the inflamed tissue where they can survive for years as indolent non-proliferative cells, manifested by high autophagy and elevated mitochondrial metabolism (termed as dormancy). Owing to several factors (cancer-cell autonomous: acquired genetic or epigenetic modifications or microenvironment dependent: local metabolic milieu or crosstalk between both), the quiescent cancer cells can “reawake” and undergo cellular differentiation (with altered genetic and epigenetic traits) that phenotypically manifests as a metastatic relapse. Among the known metabolic “support mechanisms” that cancer cells utilize to come out of dormancy in different organs are mentioned in the figure. C. Considering the parallels between reactive-stromal response and metabolic rewiring at the PMN and regenerating tissue parenchyma, it can be speculated that tumor cells can induce a paracrine loop of irreversible (and chronic) tissue inflammation and repair at the distant sites (PMN generation) to prepare for their seeding and colonization. The molecular signaling and metabolic rewiring induced at the distant sites can in turn support the indolent cancer cells to gain epigenetic traits or altered cellular states (differentiation/de-differentiation) to promote their “reawakening” from dormancy. (Index for cell types on the right; black: implicated during physiologic conditions; red: implicated during malignancy). Created with BioRender.com. ECM: Extracellular matrix, PMN: Pro-metastatic niche, VEGF: Vascular endothelial growth factor, IFN-γ: Interferon gamma, PGE2: Prostaglandin E2, ROS: Reactive oxygen species, OXPHOS: Oxidative phosphorylation, TGF-β: Transforming growth factor beta, IL-6: Interleukin-6, TANs: Tumor-associated neutrophils.
