Figure 1. Schema depicting the Insulin/IGF system.
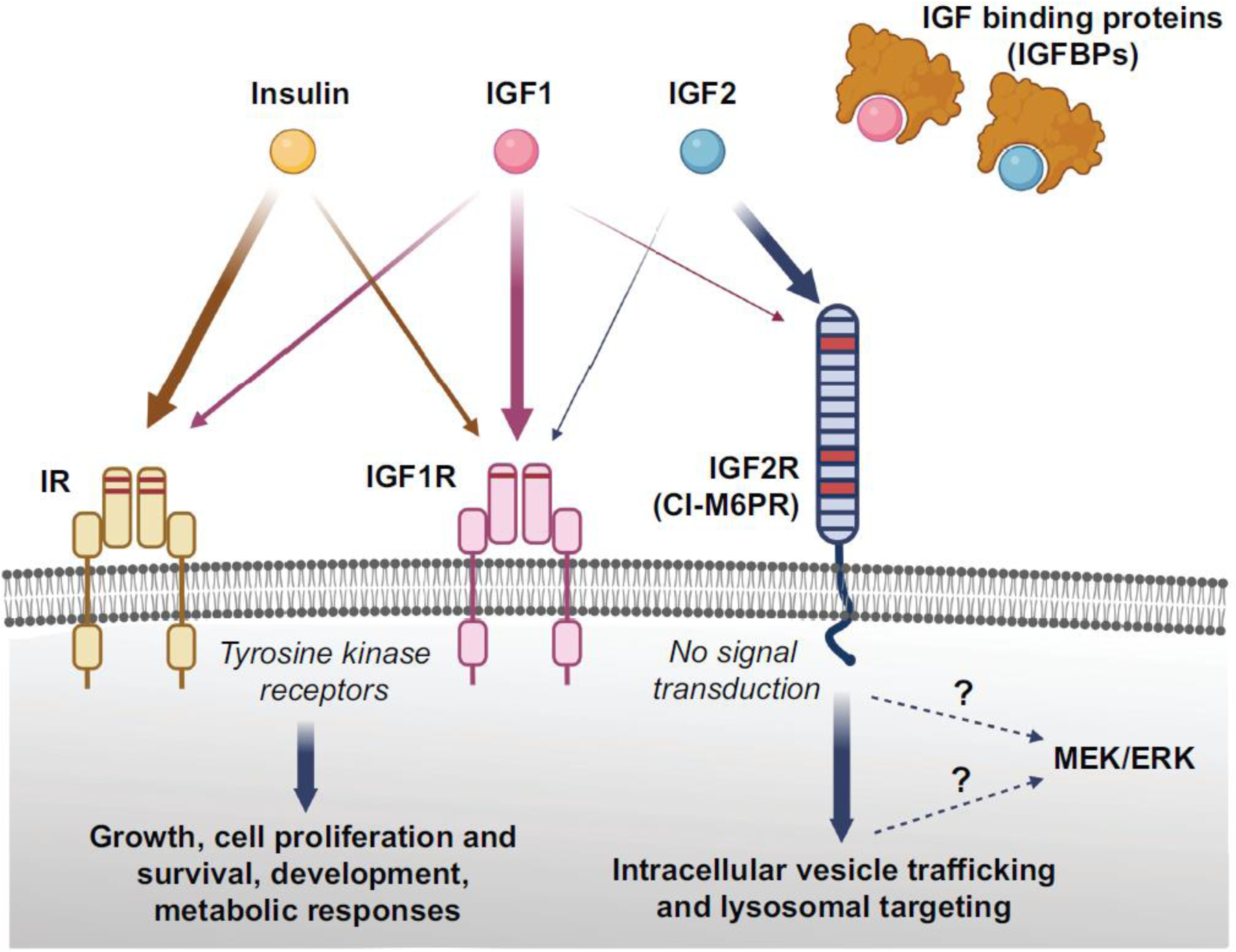
Insulin, IGF1, IGF2, and the relative high affinity receptors insulin receptor (IR), IGF1 receptor (IGF1R), and IGF2 receptor (IGF2R), along with IGF binding proteins are shown. IGF2R is also known as cation-independent mannose 6 phosphate receptor (CI-M6PR). The relative affinity of each ligand for the receptors is represented by the thickness of arrows. Insulin and IGFs can cross-bind their respective high affinity receptors, but with lower affinity, but insulin does not seem to bind to IGF2R. The IR and IGF1R are tyrosine kinase receptors; they consist of an extracellular ligand-binding domain and a cytosolic tyrosine kinase domain that autophosphorylates upon ligand binding and transphosphorylates several substrates that initiate downstream signalling supporting growth, cell proliferation and survival, development, and metabolic responses. IGF2R has a large N-terminal extracellular region of 15 homologous domains, a single membrane-spanning region, and a small cytoplasmic tail, and it lacks intrinsic signalling capability (no signal transduction). However, in neuronal cultures, Igf2/Igf2R-mediated MEK/ERK activation has been reported as critical for synapse formation and spine maturation. Whether IGF2R or downstream intermediate mechanism activate this signalling remains to be determined (indicated by the dotted lines). The binding sites for IGF2 or M6P are located on different segments of the protein. The main known functions of IGF2R are to bind IGF2, activate intracellular vesicle trafficking, and target it to lysosomal degradation, and traffic M6P-conjugated proteins, such as lysosomal enzymes. Figure created using BioRender.com
