Figure 3. Schematic model of (A) IGF2R-dependent mechanisms of action in neurons following IGF2 binding and (B) illustrating points of dysregulations that may lead to protein accumulation, and which may benefit from IGF2R ligand treatments.
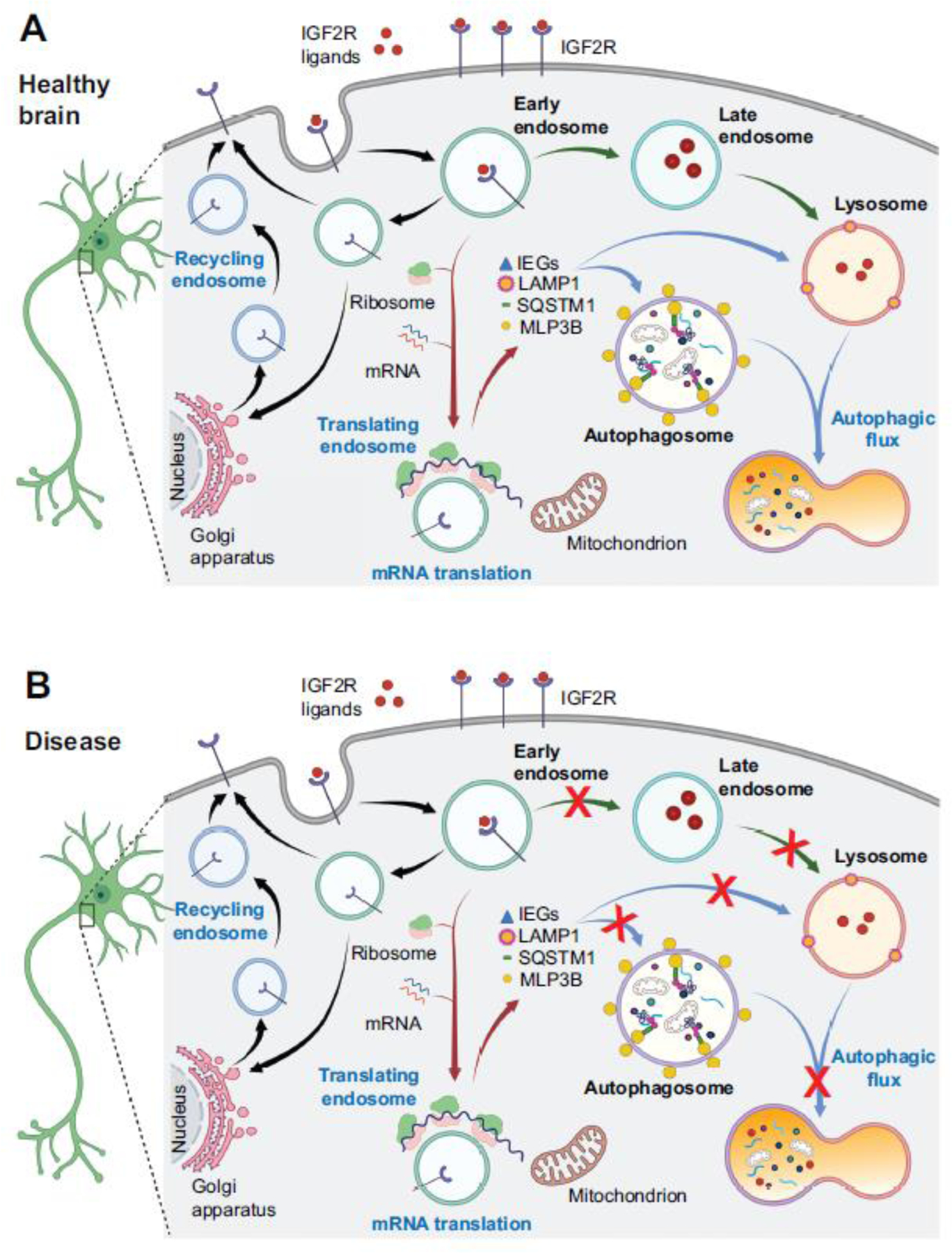
The schematic summarizes the proposed model for IGF2/IGF2R mechanisms of action in brain health and disease. (A) Binding of IGF2 to the IGF2R on the plasma membrane initiates IGF2R-bearing endosomal trafficking, beginning with the generation of early endosomes (EE). These EE can proceed to late endosomes and then lysosomes, or traffic to the Golgi apparatus, from which recycling endosomes can return to the plasma membrane. As blocking IGF2 blocks learning-induced mRNA translation [32], I hypothesize that a pool of endosomes is recruited to serve as platform for this de novo mRNA translation, including the translation of immediate early genes (IEGs) and proteins involved in autophagy such as MLP3B, SQSTM1, and LAMP1. Since learning-induced mRNA translation is coupled to autophagy [94], I suggest that the endosomes supporting translation are in direct communication with autophagosome formation and activation of autophagy. Protein degradation underlying memory and neuronal plasticity may also occur via the IGF2-bearing endosome-lysosomal classical path. Many steps and mechanisms of this model are still unclear and future studies should be able to experimentally test them. (B) A summary of possible major cell biological steps (indicated by a red X) that could be impaired in disease conditions of altered de novo protein synthesis, autophagy, and endosomal trafficking leading to protein accumulation. These diseases include neurodevelopmental disorders and neurodegenerative diseases [93, 94, 98]. Studies have shown that in brain plasticity and memory, de novo protein synthesis is coupled to autophagy [94] and that in laboratory animal models IGF2 has beneficial effects in neurodegenerative and neurodevelopmental disorders (Table 1). These results suggest that the common cell biological deficits of those diseases may include impaired autophagic flux and lysosomal targeting. The potential therapeutic-like effect produced by the activation of IGF2Rs could be in fact an increase in autophagic flux that, by enhancing protein degradation, may prevent and/or reverse protein accumulation. Figure created using BioRender.com
