Abstract
In modern cardiology, sodium‐glucose cotransporter 2 (SGLT2) inhibitors are critical components of heart failure (HF) treatment algorithms and exert their effects primarily by preventing glucose reabsorption and facilitating its urinary excretion. The objective was to systematically review randomized controlled trials (RCTs) assessing the effects of SGLT2 inhibitors, particularly canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, sotagliflozin (dual SGLT inhibitor), and their use in HF. Systematic searches of PubMed/Medline, The Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov databases were performed. There were no restrictions imposed on the date and status of publication; however, there were restrictions on language for the searched studies. A total of 1139 records were identified in the bibliographic searches from both databases and the register of choice for this systematic review. Following duplicate removal, screening for titles and abstracts, and thorough assessment of full‐text articles, 12 RCTs met the inclusion criteria. Altogether, 83 878 patients were included in this review. Among the included studies, two RCTs, with six respective reports, investigated canagliflozin, four RCTs with 13 derived reports investigated dapagliflozin, three RCTs with 12 separate reports studied the effects of empagliflozin, one RCT and its three respective reports assessed ertugliflozin's effects, and two RCTs with one added report investigated the dual inhibitor sotagliflozin. Pooled meta‐analytic effects of SGLT2 inhibitors were as follows: on atrial fibrillation odds ratio (OR) = 0.83, 95% confidence interval (CI): 0.68–1.01, prediction interval (PI): 0.57–1.19; on HF hospitalization OR = 0.69, 95% CI: 0.60–0.78, PI: 0.60–0.78; on cardiovascular death OR = 0.82, 95% CI: 0.58–1.15, PI: 0.42–1.60; and on major adverse cardiovascular events OR = 0.90, 95% CI: 0.77–1.06, PI: 0.71–1.15. SGLT2 inhibitors significantly improve the quality of life in HF patients. Their beneficial effects on HF, especially in left ventricular dysfunction, have made their use possible irrespective of diabetes mellitus or atrial fibrillation status.
Keywords: Heart failure, Sodium‐glucose transporter 2 inhibitors, Diabetes mellitus, Hypoglycaemic agents
1. Introduction
The emergence of novel therapeutic strategies for diabetes mellitus has been fuelled by the growing interest in the role of the sodium‐glucose cotransporter 2 (SGLT2), the principal protein in the proximal renal tubule responsible for reabsorbing filtered glucose. SGLT2 inhibitors are a new class of drugs that were originally designed to target glycaemic regulation in diabetes mellitus, but their scope of application is now widely acknowledged, particularly in cardiovascular disease. 1 , 2
The story of the discovery of SGLT2 inhibitors is one of serendipity, and it begins in 1835, with the isolation a naturally occurring, non‐selective SGLT inhibitor from the apple tree, known as phlorizin, which was originally used to treat malaria. 1 , 2 , 3 Thereafter, scientists discovered the glucosuric profile of phlorizin, and its consequent role in lowering plasma glucose levels. 1 , 4 A glucose molecule bound to two aromatic rings, phlorizin was recognized as a competitive inhibitor of the active transport in the kidneys, therefore resulting in excretion of both glucose and sodium in the urine. 1 , 5
From the beginning of the 21st century, several SGLT2 inhibitors were developed synthetically as phlorizin analogues, the first one being dapagliflozin in 2008, which represents an important milestone in the history. 5
On initial safety evaluation in diabetic patients, the SGLT2 inhibitors were not only found to be safe with respect to cardiovascular effects, but they also yielded other unprecedented and remarkable benefits; the use of SGLT2 inhibitors in type 2 diabetes mellitus (T2DM) patients with cardiovascular disease and reduced ejection fraction, was reported with significant reductions in cardiovascular death, non‐fatal myocardial infarction or stroke, death from any cause, preventing hospitalization for heart failure (HHF) by 25–35%, and reducing clinically relevant kidney outcomes. 1 , 2 , 5 , 6
These serendipitous findings have prompted the rapid evolution of clinical trials with SGLT2 inhibitors in patients with heart failure (HF) across the entire left ventricular ejection fraction (LVEF) range, particularly with preserved ejection fraction, as well as chronic kidney disease (CKD), both with and without diabetes mellitus, thereby greatly expanding the target population even further. 1 , 2 , 6 , 7 Indeed, SGLT2 inhibitors have become one of the most researched cardiometabolic treatments, with large‐scale randomized clinical trials completed or ongoing. 2 , 6
T2DM is associated with a two‐to‐five‐fold higher risk of developing HF. 8 Early on, in the development of HF, there are several key mechanisms that instigate functional and structural cardiac impairments, which are shared among HF and T2DM. 9 , 10 , 11 The disturbances in systemic and cardiac glucose metabolism of patients with diseases ranging from inadequate glucose management to diabetes mellitus contribute to structural and functional abnormalities of the heart, culminating in cardiac dysfunction. 12 , 13
Atrial fibrillation (AF) is another condition that coexists with HF owing to common risk factors including valvular disease, hypertension, age, and diabetes, and is associated with a three‐fold higher risk of incidence of HF. 14 , 15 Neurohormonal, electrophysiological and myocardial cellular maladaptive alterations are mechanistic contributors to elevated filling pressures and increased afterload, therefore predisposing the heart to failure as well as AF. In patients with systolic HF, AF is a poor prognostic indicator with a potential to alter the therapeutic effects. Considering that in individuals with HF and reduced ejection fraction, AF invokes unfavourable cardiovascular outcomes and that non‐antiarrhythmic drugs have already revealed their potential in reducing AF rates in these patients, elucidation of the interrelation between HF and AF has also sparked an interest in research through treatment effects of SGLT2 inhibitors. 14
SGLT2 inhibitors' mechanisms of action of in HF are still a matter of conjecture, even though the drugs exhibit several metabolic, haemodynamic, and organ‐specific effects; however, it is unlikely that prevention and treatment of HF are exclusively due to the favourable metabolic and haemodynamic effects. 16 , 17 Another mode though which SGLT2 inhibitors incite their beneficial effects is by inhibiting of the sodium‐hydrogen exchanger (NHE1) activity, which is up‐regulated both in T2DM and HF; inhibition of NHE1 receptors provides protection of the heart from toxic intracellular calcium ion (Ca2+) overload. 18 SGLT2 inhibitors may also exert direct effects on myocardial metabolism and decrease myocardial oxidative stress. 19 Furthermore, by promoting a metabolic shift from free fatty acid (FFA) to glucose oxidation, SGLT2 inhibitors result in increased cardiac adenosine triphosphate (ATP) production, preventing a decrease in cardiac function. 12
The SGLT2 inhibitors share similar pharmacokinetic properties, including a rapid oral absorption, a long half‐life which grants the possibility for once‐daily administration, extensive hepatic metabolism to inactive metabolites primarily via glucuronidation, and low renal elimination. 20 Additionally, due to the glycoside structure of the SGLT2 inhibitors, there are several pharmacokinetic issues to account for, including poor stability, low tissue permeability, and a possibility of drug interactions. 17 , 21
The pharmacodynamic effect of SGLT2 inhibitors is exhibited by inducing glycosuria though a decrease in the threshold for glucose resorption. SGLT2 inhibitors differ pharmacodynamically, thus resulting in different beneficial and adverse effect profiles (Table 1 ). 17 Drugs in this class may result in a range of adverse effects, including an increased risk of genitourinary infections (due to the high glucose concentration in the genitourinary tract, and the disturbed function of neutrophils and the antioxidant system resulting in an impairment of the immune system), postural hypotension (they display an osmotic diuretic effect, leading to slight volume depletion, mainly due to glucose and sodium depletion), polyuria, acute kidney injury (AKI), diabetic ketoacidosis (due to the decreased insulin levels following loss of glucose in the urine; thus, SGLT2 inhibitors' use is contraindicated in diabetes mellitus type 1 patients), as well as bone fractures (as it decreases bone mineral density due to effects on phosphate, calcium, and vitamin D) or lower limb amputations (particularly with canagliflozin, and in individuals with a history of peripheral vascular disease). 17 , 20 , 21 , 22 Pharmacological differences between individual SGLT2 inhibitors rely on the selectivity of SGL2 versus SGLT1, other potential differences remain incompletely understood, mainly due to the lack of head‐to‐head trials. 17
Table 1.
Pharmacological properties of SGLT2 inhibitors: dapagliflozin, empagliflozin, canagliflozin, ertugliflozin, and sotagliflozin
| Characteristics | Dapagliflozin | Empagliflozin | Canagliflozin | Ertugliflozin | Sotagliflozin |
|---|---|---|---|---|---|
| Route of administration | PO | PO | PO | PO | PO |
| Dosage (mg) | 5 and 10 | 10 and 25 | 100 and 300 | 5 and 15 | 200 and 400 |
| Mechanism of action | SGLT2 inhibitor | SGLT2 inhibitor | SGLT2 inhibitor | SGLT2 inhibitor | SGLT1 and SGLT2 (dual) inhibitor |
| Metabolism | UGT1A9 |
UGT2B7 UGT1A3 UGT1A8 UGT1A9 |
UGT1A9 UGT2B4 |
UGT1A9 UGT2B7 CYP3A4 CYP3A5 |
UGT1A9 UGT1A1 UGT2B7 CYP3A4 |
| Excretion |
75% renal 21% faecal |
55% renal 40% faecal |
41.5% faecal 33% renal |
50% renal 41% faecal |
Mostly renal |
| Bioavailability (%) | ~78 | ~75 | ~65 | 70–90 | 71 |
| Half‐life (h) | 1–1.5 or 13 | 1.5 or 13 | 1–2 or 11–13 | 0.5–1.5 or 11–17 | 3 or 13.5–20.7 |
| Indications |
HFrEF DMT2 CKD |
HFrEF HFpEF DMT2 |
DMT2 | DMT2 |
Not yet FDA‐approved for heart failure and kidney disease Added to insulin therapy in adults with DMT1 for glycaemic control |
| Contraindications |
Hypersensitivity Severe renal impairment, ESRD, or dialysis |
Hypersensitivity Severe renal impairment, ESRD, or dialysis |
Hypersensitivity Severe renal impairment, ESRD, or dialysis |
Hypersensitivity Severe renal impairment, ESRD, or dialysis |
Hypersensitivity Severe renal impairment, ESRD, or dialysis |
| Renal impairment | eGFR <60 mL/min/1.73 m2: contraindicated | eGFR <45 mL/min/1.73 m2: contraindicated |
eGFR <60 and >45 mL/min/1.73 m2: dose reductions required eGFR of <45 mL/min/1.73 m2: contraindicated |
No dose adjustment needed |
eGFR <60 mL/min/1.73 m2: not recommended eGFR is persistently <45 mL/min/1.73 m2: discontinued |
| Drug interactions | No significant with other drugs commonly used in T2DM | No significant with other drugs commonly used in T2DM | No significant with other drugs commonly used in T2DM | No significant with other drugs commonly used in T2DM | No significant with other drugs commonly used in T2DM |
CKD, chronic kidney disease; CYP, cytochrome P‐450 enzymes; DMT1, diabetes mellitus type 1; DMT2, diabetes mellitus type 2; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PO, per os; SGLT1, sodium‐glucose cotransporter‐1; SGLT2; sodium‐glucose cotransporter 2; UGT, uridine 5′‐diphospho‐glucuronosyl transferase.
2. Methods
A systematic review of all studies on SGLT2 inhibitors' effects in patients with HF with reduced, mildly reduced, and preserved ejection fraction was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement, and the Cochrane Handbook for Systematic Reviews of Interventions.
2.1. Search methods for identification of studies
Electronic searches of PubMed/Medline, The Cochrane Central Register of Controlled Trials (CENTRAL) databases were performed, as well as grey literature obtained from ClinicalTrials.gov was searched, as an attempt to avoid steering towards positive results only and reducing risk of bias. The terms used for the research included the following: “Heart Failure,” “Sodium‐Glucose Transporter 2 Inhibitors,” “Diabetes Mellitus,” which were combined in multiple ways to generate an extensive search strategy through the MeSH database, with attention to transparency along the process (see Appendix S1 – S3 for detailed search strategies). No restrictions were imposed on the date and status of publication. There were, however, restrictions on language for the searched studies.
The rationale to perform this systematic review and the methodology that was used were determined with a well‐defined guiding research statement, which included the PICO elements (Population, Intervention, Comparison, Outcome) matching with the HF patients with or without diabetes, SGLT2 inhibitors as interventions, compared with placebo, and endpoints of major adverse cardiovascular events (MACE), hospitalization for heart failure (HHF), as well as renal outcomes, respectively, in the present study. Once the PICO question was developed, clear inclusion and exclusion criteria for the studies of interest were determined. Criteria for considering studies for this systematic review were based on the types of studies, types of participants, interventions, and outcomes of interest.
2.2. Inclusion criteria
2.2.1. Population
Adult patients (>18 years up to 80 years of age), both men and women with HF with reduced (LVEF <40%), mildly reduced (LVEF 40–49%), and preserved (LVEF >50%) ejection fraction, diabetic and non‐diabetic patients.
2.2.2. Interventions
Only canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, and sotagliflozin were the drugs of choice to be included in this study.
2.2.3. Types of studies
Only randomized controlled trials (RCTs), studies about humans, published in English language, were included, as well as the analysis of each article being consistent with the PICO study question.
2.2.4. Outcomes of interest
RCTs with the following intended outcomes were eligible for this study: MACE, defined as a composite of cardiovascular death, non‐fatal myocardial infarction (MI), and non‐fatal stroke; and HHF. Other outcomes were renal function in terms of sustained decline in eGFR, end‐stage renal disease, or serum creatinine; all‐cause mortality; reduction in HbA1c; and side effects such as amputation, urinary tract infection, or risk of hypoglycaemia. In addition, AF as a serious adverse event in HF patients treated with SGLT2 inhibitors was one of the selected outcomes of interest for the meta‐analysis.
2.3. Exclusion criteria
Records in which SGLT2 inhibitors (gliflozins) other than dapagliflozin, empagliflozin, canagliflozin, ertugliflozin, and sotagliflozin were mentioned in the titles and abstracts were excluded. Furthermore, records where the drugs of interest were investigated in relation to outcomes that did not meet inclusion criteria of this review, and SGLT2 inhibitor trials in which the primary endpoint measured was the quality of life based on The Kansas City Cardiomyopathy Questionnaire (KCCQ), were also excluded. Other exclusion criteria were as follows: records not mentioning HF, other reviews were not included, any records that were irrelevant to the main PICO question, records referring to other topics that were not related to cardiovascular disease or HF, and animal studies.
2.4. Data collection and analysis
2.4.1. Selection of studies
All titles and abstracts identified in the bibliographic searches were screened by five authors with the use of the Rayyan free web tool. This process was followed by duplicate detection and removal. Full‐text articles were then retrieved manually and assessed for potentially relevant studies.
2.4.2. Data extraction
Data were manually extracted from the included studies on study design, patient characteristics, follow‐up durations, intervention, comparison, baseline characteristics, and results regarding the related outcomes of interest with different drugs relevant to our review. Any missing data were found in ClinicalTrials.gov for the included RCTs.
Where results from a single trial were reported in more than one article, the most complete publication was preferred. If deemed relevant for the purpose of this study based on the established criteria, selected reports and analyses were also included.
Results of treatment effects from the included studies were reported and interpreted in terms of hazard ratios (HR), with the corresponding 95% confidence intervals (CI) and P‐value for statistical significance of the findings, illustrated in Tables 2 , 3 , 4 , 5 , and 6 .
Table 2.
Results of RCTs investigating canagliflozin in terms of cardiovascular outcomes of interest
| Trial name | CANVAS | CREDENCE |
|---|---|---|
| Number of participants | 10 142 | 4401 |
| Intervention | Canagliflozin | Canagliflozin |
| Dosing (once daily) | 100 or 300 mg | 100 mg |
| Mean age (years) | 63 | 63 |
| Median follow‐up (months or years) | 3.6 years | 2.62 years |
| Baseline HbA1c (%) | ≥7.0 and ≤10.5 | ≥6.5 and ≤12 |
| Baseline eGFR(mL/min/1.732) | >30 | 30 to <90 |
| Mean LVEF (%) |
<50 >50 |
<50 |
|
Cardiovascular effects: MACE/HHF |
3‐MACE: HR 0.86 (95% CI: 0.75–0.97; P < 0.001 for non‐inferiority; P = 0.02 for superiority) |
3‐MACE: HR 0.80 (95% CI: 0.67–0.95; P = 0.01) HHF: HR 0.61 (95% CI: 0.47–0.80; P < 0.001) Composite of end‐stage kidney disease: HR 0.70 (95% CI: 0.59–0.82; P = 0.00001) |
CANVAS, The Canagliflozin Cardiovascular Assessment Study; CI, confidence interval; CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV, cardiovascular; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiac events.
Table 3.
Results of randomized controlled trials investigating dapagliflozin in terms of cardiovascular outcomes of interest
| Trial name | DECLARE‐TIMI 58 | DAPA‐HF | DAPA‐CKD | DELIVER |
|---|---|---|---|---|
| Number of participants | 17 190 | 4744 | 4304 | 6263 |
| Intervention | Dapagliflozin | Dapagliflozin | Dapagliflozin | Dapagliflozin |
| Dosing (once daily) | 10 mg | 10 mg or 5 mg | 10 mg | 10 mg |
| Mean age (years) | 64 | 66 | 62 | 72 |
| Median follow‐up (months or years) | 4.2 years | 18.2 months | 2.4 years | 2.3 years |
| Baseline HbA1c (%) | 6.5 − 12.0 | 8.3 | 6.5 | 6.6 |
| Baseline eGFR (mL/min/1.732) | >60 | ≥30 | 25–75 | 61 |
| Mean LVEF (%) | <45 | ≤40 | N/A | >40 |
| Cardiovascular effects: MACE/HHF | 3‐MACE: HR 0.83 (95% CI: 0.73–0.95; P = 0.005) | CV death, hospitalization for HF, or urgent HF visit: HR 0.75 (95% CI: 0.65–0.86, P < 0.0001) | Risk of the primary endpoint (sustained ≥50% eGFR decline, ESKD) in patients with HF: HR 0.58 (95% CI: 0.37–0.91) | Composite of worsening HF or CV death: HR 0.82 (95% CI: 0.73–0.92, P < 0.001) |
CI, confidence interval; CV, cardiovascular; DAPA‐CKD, Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease; DAPA‐HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DECLARE‐TIMI 58, Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events; DELIVER, Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure; DM, diabetes mellitus; ESKD, end‐stage kidney disease; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiac events; N/A, not available.
Table 4.
Results of randomized controlled trials investigating empagliflozin in terms of cardiovascular outcomes of interest
| Trial name | EMPA‐REG OUTCOME | EMPEROR‐Reduced | EMPEROR‐Preserved |
|---|---|---|---|
| Number of participants | 7064 | 3730 | 5988 |
| Intervention | Empagliflozin | Empagliflozin | Empagliflozin |
| Dosing (once daily) | 10 mg or 25 mg | 10 mg | 10 mg |
| Mean age (years) | 63.1 | 67 | 72 |
| Median follow‐up (months or years) | 3.1 years | 16 months | 2.2 years |
| Baseline HbA1c (%) | 7–10 | 5–12 | 6.3–7.8 |
| Baseline eGFR (mL/min/1.732) | ≥30 |
20 *The rate of decline in eGFR in patients with diabetes was nearly twice that in patients without diabetes |
<60 or ≥60 |
| Mean LVEF (%) | N/A | 28 | 54 |
| Cardiovascular effects: MACE/HHF | 3‐MACE: HR 0.86 (95.02% CI: 0.74–0.99; P < 0.001 for non‐inferiority and P = 0.04 for superiority) | CV death or HF hospitalization: HR 0.75 (95% CI: 0.65–0.86, P < 0.001) | Combined risk of CV death or HF hospitalization: HR 0.79 (95% CI: 0.69–0.90; P < 0.001) |
CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; EMPA‐REG, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; EMPEROR‐Reduced, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; EMPEROR‐Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure With Preserved Ejection Fraction; HHF, hospitalization for heart failure; HR, hazard ratio; MACE, major adverse cardiac events; N/A, not available.
Table 5.
Results of randomized controlled trials investigating ertugliflozin in terms of cardiovascular outcomes of interest
| Trial name | VERTIS‐CV |
|---|---|
| Number of participants | 8246 |
| Intervention | Ertugliflozin |
| Dosing (once daily) | 5 mg or 15 mg |
| Mean age (years) | 64 |
| Median follow‐up (months or years) | 3.5 years |
| Baseline HbA1c (%) |
7.0 to 10.5 *greater reductions in HbA1c |
|
Baseline eGFR (mL/min/1.732) |
≥30 *34% reduction in risk of decline in eGFR |
| Mean LVEF (%) | >40 |
|
Cardiovascular effects: MACE/HHF |
Rates of MACE: ~4% per year 3‐MACE: HR 0.97 (95.6% CI: 0.85–1.11; P < 0.001 for non‐inferiority) |
CI, confidence interval; CV: cardiovascular; DM: diabetes mellitus; HF: heart failure; HR: hazard ratio; MACE: major adverse cardiac events; VERTIS‐CV, Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial.
Table 6.
Results of randomized controlled trials investigating sotagliflozin in terms of cardiovascular outcomes of interest
| Trial name | SOLOIST‐WHF | SCORED |
|---|---|---|
| Number of participants | 1222 | 10 584 |
| Intervention | Sotagliflozin | Sotagliflozin |
| Dosing (once daily) | 200 mg (up to 400 mg) | 200 mg (up to 400 mg) |
| Mean age (years) | 70 | 70 |
| Median follow‐up (months or years) | 9 months | 24 months |
| Baseline HbA1c (%) | 7.1 | 8.3 |
| Baseline eGFR (mL/min/1.732) | 49.7 | 25–60 |
| Mean LVEF (%) |
35 <50 79 |
60 |
|
Cardiovascular effects: MACE/HHF |
Total CV death, hospitalization for HF, or urgent visit for HF: HR 0.67 (95% CI: 0.52–0.85; P < 0.001) |
Original co‐primary endpoint of the first occurrence of 3‐MACE: HR 0.84 (95% CI: 0.72–0.99, P = 0.035) Changed primary endpoint to CV death, HF hospitalization, urgent visit for HF: HR 0.74 (95% CI: 0.63–0.88; P < 0.001) |
CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiac events; SCORED, Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk; SOLOIST‐WHF, Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure.
2.5. Assessment of risk of bias in included studies
The internal validity and critical appraisal of the included RCTs were based on an assessment of trial publications and protocols. Risk‐of‐bias (RoB) assessment was performed by means of the Cochrane Risk of Bias tool (version 5.2). In each trial, namely, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR‐Reduced), Empagliflozin Outcome Trial in Patients with Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR‐Preserved), Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA‐REG OUTCOME), Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA‐HF), Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE‐TIMI 58), Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA‐CKD), The Canagliflozin Cardiovascular Assessment Study (CANVAS), Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial (VERTIS‐CV), Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST‐WHF), Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED), and Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER), risk‐of‐bias judgements labelled as either low, high or unclear, were made for the following five domains: (i) random sequence generation (selection bias); (ii) allocation concealment (selection bias); (iii) blinding of outcome assessment (detection bias); (iv) incomplete outcome data (attrition bias); and (v) selective reporting (reporting bias).
2.6. Meta‐analysis
For the meta‐analysis, one of the chosen outcomes of interest was based on the serious adverse events reported in the primary studies, with a particular focus on AF occurrence in HF patients treated with SGLT2 inhibitors. Two studies assessing sotagliflozin were excluded from the meta‐analysis, given that SOLOIST‐WHF primarily included acute HF patients, and like the SCORED trial, both were prematurely terminated due to loss of funding as well as the COVID‐19 pandemic; the recent DELIVER trial was also excluded from the meta‐analysis, as data for these three trials with respect to AF occurrence were not available.
Moreover, the CANVAS Program study data on AF was collected separately for CANVAS and CANVAS‐R because of the different trial aims, as well as considering that they were phase 3 and phase 4 trials, respectively. Data for canagliflozin in the CANVAS trial, empagliflozin in the EMPA‐REG OUTCOME trial, and ertugliflozin in the VERTIS‐CV trial were collected for the two doses together. The rest of the data were collected for the single doses of the interventions used in the included trials (EMPEROR‐Preserved, EMPEROR‐Reduced, DAPA‐HF, DAPA‐CKD, DECLARE‐TIMI 58, CREDENCE). Numbers of included RCTs registered at ClinicalTrials.gov registry can be found in Appendix S4 .
In addition, a meta‐analysis of common side effects of SGLT2 inhibitors was conducted, as well as a comparison of the effects of SGLT2 inhibitors including above‐mentioned trials, glucagon‐like‐peptide‐1 (GLP‐1) agonist trials Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE‐O), The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND), Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL), and Trial to Evaluate Cardiovascular and Other Long‐term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN‐6), and dipeptidyl peptidase‐4 (DPP‐4) inhibitor trials Cardiovascular Safety and Renal Microvascular Outcome Study with Linagliptin (CARMELINA), Study to Assess Cardiovascular Outcomes Following Treatment With Omarigliptin (MK‐3102) in Participants with Type 2 Diabetes Mellitus (OMNEON), Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS), and The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction (SAVOUR‐TIMI 53). Other trials of these groups of drugs were excluded either due to unavailability of data or differing definitions of the intended endpoints (some were documented as primary outcomes, secondary outcomes, or as adverse events).
2.6.1. Statistical analysis
Meta‐Essentials tool for meta‐analysis (Suurmond R, van Rhee, H, Hak T, 2017) was used for all statistical analyses. For the cardiovascular serious adverse event of interest, in this case, AF occurrence, risk ratio, and 95% CIs were meta‐analysed using a random‐effects model; the effect size measure was presented as odds ratio (OR). Also, a random‐effects model was selected to account for heterogeneity in the included studies. In addition, inverse variance was chosen as a weighting method.
To generate a combined effect size regarding AF following SGLT2 inhibitor use, as well as common side effects, the overall study population in this meta‐analysis included 65 677 HF patients (n = 36 120 in the SGLT2 inhibitor arms; n = 29 557 in the placebo arms). In the analysis comparing the three groups of drugs, 36 827 patients for SGLT2 inhibitors, 32 026 patients for GLP‐1 agonists, and 42 334 patients for DPP‐4 inhibitors were included.
To assess whether publication bias affected the meta‐analysis, a funnel plot and Egger's regression test were performed; P‐value ≤0.05 was defined as statistically significant.
3. Results
3.1. Study selection
A total of 1139 records were identified in the bibliographic searches from both databases and the register of choice for this systematic review. Of these, following duplicates removal and screening for titles and abstracts, 184 titles and abstracts potentially met the pre‐specified review inclusion criteria, and the full‐text articles were retrieved and assessed thoroughly. One hundred thirty‐eight trials were excluded, and 12 RCTs met the inclusion criteria. The study selection process is summarized in Figure 1 , based on the 2020 version of the PRISMA flow diagram.
Figure 1.
PRISMA 2020 Flow Diagram.
3.2. Included studies
The characteristics of the included studies and selected reports of those trials for each of the drugs (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin and sotagliflozin) included in this review are clearly illustrated in Tables 7 , 8 , 9 , 10 , and 11 . The parallel group randomized clinical trials included, were published between 2015 and 2021, and they were conducted in high‐income countries.
Table 7.
Characteristics of included studies and their reports for canagliflozin
| Author | Title | Journal | Methods | Findings |
|---|---|---|---|---|
|
Perkovic et al. (2019) (CREDENCE Trial) |
Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy |
The New England Journal of Medicine |
4401 type 2 diabetic patients with albuminuric chronic kidney disease, and an eGFR 30 to <90 mL/min/1.73 m2 were randomized canagliflozin 100 mg or placebo. In this double‐blinded trial, composite of ESKD, doubling of creatinine, or death from renal or CV causes were assessed. |
Patients on canagliflozin showed 30% decreased relative risk of the main outcome. ESKD was associated with a relative risk reduction of 32%, compared with a relative risk reduction of 34% for the renal‐specific composite of creatinine doubling or death from renal causes. The incidence of fractures or amputations did not differ statistically significantly. |
|
Neal et al. (2017) (CANVAS Program) |
Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes |
The New England Journal of Medicine |
10 142 men and women with T2DM, a history of symptomatic ASCVD, and an eGFR >30 mL/min/1.73 m2 were randomized to canagliflozin 100 mg or 300 mg, or placebo. Aim was to measure a composite of death from cardiovascular causes, non‐fatal myocardial infarction, or non‐fatal stroke, progression of albuminuria, and serious adverse events. |
The canagliflozin group was at a greater risk of amputation but experienced considerably fewer primary outcome events than the placebo group. Additionally, progression of albuminuria and the cumulative sustained 40% decline in eGFR occurred less frequently with canagliflozin treatment than with placebo. |
| Reports from CANVAS and CREDENCE | ||||
| Yu et al. (2021) | Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS Program | ESC Heart Failure | A post hoc subgroup analysis of the CANVAS Program, in which patients were classified based on their baseline diuretic use, such as loop diuretics, MRAs, thiazides, and ASCVD or HF. | Patients on baseline diuretic medication, who received canagliflozin, exhibited a greater relative risk reduction in MACE than those not on diuretics. |
| Sarraju et al. (2021) | Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: Results from the CREDENCE trial | American Heart Journal | Participants from the CREDENCE trial underwent investigation on whether canagliflozin safely decreases CV and renal events in those with T2DM and nephropathy, with and without previous HF history prior to randomization. | Canagliflozin reduced cardiovascular and renal events in patients with and without a history of heart failure; the results support the safety and efficacy of canagliflozin in individuals with T2DM and nephropathy independently of HF history. |
| Januzzi et al. (2020) |
Effects of Canagliflozin on Amino‐Terminal Pro–B‐Type Natriuretic Peptide Implications for Cardiovascular Risk Reduction |
Journal of the American College of Cardiology | A post hoc subgroup analysis of the CANVAS Program, which aimed to identify associations between baseline NT‐proBNP and cardiovascular, renal, and mortality outcomes related to canagliflozin. | Canagliflozin lowered NT‐proBNP levels by 11%, accounting for only a modest amount of its effectiveness in HF events. Participants with elevated NT‐proBNP were at a higher risk of cardiovascular events. |
| Figtree et al. (2019) | Effects of Canagliflozin on Heart Failure Outcomes Associated With Preserved and Reduced Ejection Fraction in Type 2 Diabetes Mellitus Results From the CANVAS Program | Circulation | In participants from CANVAS Program, the effect of canagliflozin across the broad spectrum of HF patients, including those with HFpEF and HFrEF, was examined. | Canagliflozin reduced the HF event risk in patients with T2DM and high cardiovascular risk, with no marked difference between HFrEF and HFpEF events. |
| Rådholm et al. (2018) | Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus Results From the CANVAS Program | Circulation | Patients from CANVAS Program were followed for 188 weeks for adjudicated CV death or HHF. | Canagliflozin decreased the risk of cardiovascular death or hospitalized HF in vast scope of patient subgroups with T2DM and a high risk of CV disease; these benefits were greater in individuals with baseline HF history. |
| Perkovic et al. (2018) | Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomized clinical trials | The Lancet Diabetes & Endocrinology | A pre‐specified exploratory analysis of CANVAS Program participants, which aimed to report changes in UACR, ESKD, doubling of serum creatinine, annual eGFR reductions, and death from renal causes. | Treatment with canagliflozin was linked to a lower risk of long‐term kidney damage, slowed eGFR decline, and decreased albuminuria, which suggested that this medication may have renoprotective effects in type 2 diabetics. |
ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end‐stage kidney disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular events; MRAs, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; T2DM, type 2 diabetes mellitus; UACR, urinary albumin‐to‐creatinine ratio.
Table 8.
Characteristics of included studies and their reports for dapagliflozin
| Author | Title | Journal | Methods | Findings |
|---|---|---|---|---|
|
Solomon et al. (2022) (DELIVER Trial) |
Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction | The New England Journal of Medicine | 6263 patients with HF and LVEF >40% (HF with mildly reduced [HFmrEF] and preserved [HFpEF] ejection fractions) participated in the trial, and were randomized to dapagliflozin 10 mg or placebo, along with usual therapy. The primary outcome assessed was a composite of worsening HF, defined as unplanned HHF or urgent visit for HF or CV death. | Treatment of HF patients with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF) with dapagliflozin yielded an 18% risk reduction in the composite of worsening HF or cardiovascular death, regardless of presence or absence of diabetes. Additionally, there was a similar incidence of adverse events in patients with LVEF ≥60% and those with LVEF <60%. This effect of dapagliflozin was consistent, suggesting that this SGLT2 inhibitor is beneficial in these groups of HF patients, in whom limited therapies are available. |
|
Heerspink et al. (2020) (DAPA‐CKD Trial) |
Dapagliflozin in Patients with Chronic Kidney Disease | The New England Journal of Medicine | The trial involved 4304 participants with eGFR 25 to 75 mL/min/1.73 m2 and a UACR of 200 to 5000, who were randomized to dapagliflozin 10 mg or placebo. Sustained decline in the eGFR of at least 50%, ESKD, or death from renal or CV causes, were evaluated as a composite of the primary outcome. | Primary outcome event occurred in 9.2% of patients in the dapagliflozin group and 14.5% in the placebo group. Dapagliflozin treatment resulted in a significant 39% risk reduction in the composite of a sustained 50% decline in the eGFR, ESKD, or death from renal or cardiovascular causes among patients with chronic kidney disease, irrespective of diabetes status. |
|
McMurray et al. (2019) (DAPA‐HF Trial) |
Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction | The New England Journal of Medicine | 4744 patients with NYHA class II, III, or IV HF and a LVEF ≤40%, received either dapagliflozin 10 mg or placebo, in addition to recommended therapy. Worsening HF was the primary composite outcome that was evaluated, defined as hospitalization or an urgent visit followed by intravenous therapy for HF, or CV death. | With dapagliflozin treatment, the risk of the key composite outcome of worsening HF was reduced by 30%, a finding that was comparable in diabetic and non‐diabetic patients. |
|
Wiviott et al. (2019) (DECLARE‐TIMI 58 Trial) |
Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes | The New England Journal of Medicine | 17 160 patients with T2DM who had ASCVD or were at risk for developing it were randomized to dapagliflozin or placebo. MACE, defined as CV death, myocardial infarction, or ischaemic stroke, was the primary safety outcome assessed. | There was no difference between dapagliflozin treatment and placebo in the rate of MACE in patients with T2DM with or at risk for ASCVD, but it did lead to reductions in the rate of CV death or HHF; this finding reveals a lower rate of hospitalization for HF. |
| Reports from DAPA‐HF, DECLARE‐TIMI 58, and DAPA‐CKD | ||||
| Heerspink et al. (2022) | A pre‐specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA‐CKD) randomized controlled trial on the incidence of abrupt declines in kidney function | Kidney International | Participants from DAPA‐CKD trial were assessed for an abrupt decline in kidney function in terms of serum creatinine doubling between two subsequent visits and AKI‐related adverse events. | The risk of a sudden deterioration in kidney function was reduced with dapagliflozin treatment in patients with CKD and substantial albuminuria, whereby doubling of serum creatinine occurred in 22.9% of patients. Serious adverse events linked to AKI occurred in 2.5% of patients receiving dapagliflozin, with no discernible difference between the two groups. |
| Berg et al. (2021) | Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction | JAMA Cardiology | In participants from DAPA‐HF trial, the timing of onset of clinical benefit with dapagliflozin as compared with placebo was examined, and the timing of the most recent HF hospitalization was considered to categorize patients. | Dapagliflozin therapy was linked to a fast decline in the risk of cardiovascular death or worsening HF (by 49%), with a sustained statistically significant benefit emerging very early, 28 days after randomization. |
| McMurrary et al. (2021) | Effects of Dapagliflozin in Patients With Kidney Disease, With and Without Heart Failure | JACC: Heart Failure | In DAPA‐CKD trial patients, who were randomized to dapagliflozin 10 mg and placebo, composite endpoint of ≥50% decline in eGFR, ESKD, and cardiovascular or renal death was assessed. | Regardless of a history of HF, dapagliflozin improved survival in CKD patients with or without T2DM by lowering the risk of renal failure, CV death, or HHF. |
| Zelniker et al. (2021) | Effect of Dapagliflozin on Cardiovascular Outcomes According to Baseline Kidney Function and Albuminuria Status in Patients With Type 2 Diabetes A Prespecified Secondary Analysis of a Randomized Clinical Trial | JAMA Cardiology | In participants from DECLARE‐TIMI 58 trial, the cardiovascular efficacy and safety of dapagliflozin were assessed using the baseline eGFR and UACR. | Dapagliflozin's effect on the relative risk for CV events was consistent regardless of kidney function or albuminuria status; patients with a lower eGFR and albuminuria experienced absolute major benefit regarding CV death or HHF composite. |
| Docherty et al. (2021) | Extrapolating Long‐term Event‐Free and Overall Survival With Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction An Exploratory Analysis of a Phase 3 Randomized Clinical Trial | JAMA Cardiology | In participants from DAPA‐HF trial, the long‐term treatment effects of dapagliflozin in HFrEF patients over the duration of a patient's lifetime were estimated, with primary composite outcome being time to first HHF, urgent HF visit followed by intravenous therapy, or CV death. | With dapagliflozin, the estimated event‐free and overall survival times were longer (8.3 years) compared with placebo (6.2 years), suggesting that dapagliflozin provides clinically meaningful gains in extrapolated event‐free and overall survival in HFrEF patients. |
| Butt et al. (2021) | Efficacy and Safety of Dapagliflozin in Men and Women With Heart Failure With Reduced Ejection Fraction A Prespecified Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial | JAMA Cardiology | In DAPA‐HF trial participants, the efficacy and safety of dapagliflozin was investigated in men versus women with HFrEF, with the composite of an episode of worsening HF (HF hospitalization or urgent HF visit requiring intravenous therapy) or CV death. | Dapagliflozin was safe, well‐tolerated, reduced the risk of worsening HF, CV death, and all‐cause mortality, and was effective in improving symptoms, physical function, and health‐related quality of life comparably in men and women with HFrEF. Therefore, the effect of dapagliflozin was consistent in both men and women. |
| Solomon et al. (2020) |
Effect of Dapagliflozin in Patients With HFrEF Treated With Sacubitril/Valsartan The DAPA‐HF Trial |
JACC: Heart Failure | Patients from DAPA‐HF trial were analysed according to whether they were taking sacubitril/valsartan at randomization. Dapagliflozin's efficacy on the primary composite outcome, defined as CV death or episode of worsening HF, its components, and all‐cause mortality, was evaluated. | In patients receiving sacubitril/valsartan and those not on it, dapagliflozin was equally safe and effective (25% risk reduction) in the DAPA‐HF trial, indicating that combining the two medications could further decrease morbidity and mortality in HFrEF patients. |
| Docherty et al. (2020) | Effect of Dapagliflozin on Outpatient Worsening of Patients With Heart Failure and Reduced Ejection Fraction A Prespecified Analysis of DAPA‐HF | Circulation | In participants from DAPA‐HF trial, the frequency and significance of episodes of outpatient HF worsening, requiring the augmentation of oral therapy, and the effects of dapagliflozin on these additional events were assessed. | With dapagliflozin, there was a 27% risk reduction in participants who experienced the expanded outcome in DAPA‐HF compared with placebo. Outpatient episodes of HF worsening were common, were of prognostic importance, and were reduced by dapagliflozin. |
| Zelniker et al. (2020) | Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without sodium–glucose co‐transporter 2 inhibitor therapy in DECLARE‐TIMI 58 | European Journal of Heart Failure | In DECLARE‐TIMI 58 trial participants, baseline NT‐proBNP and hsTnT levels were measured. | In HFrEF patients, dapagliflozin reduced the relative risk of CV death/HHF by 17%, irrespective of NT‐proBNP and hsTnT levels, with greater absolute risk reductions seen in those with higher baseline biomarker levels. |
| Kato et al. (2019) | Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus | Circulation | In participants from DECLARE‐TIMI 58 trial, baseline HF status stratified by LVED, and composite of CV death, HHF, and all‐cause mortality were assessed. | Dapagliflozin reduced HHF in individuals with and without HFrEF and decreased CV death and all‐cause mortality in those with HFrEF by 38% in this study, the first SGLT2 inhibitor cardiovascular outcome trial to analyse patients with T2DM stratified by EF. |
| Mosenzon et al. (2019) | Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomized trial | The Lancet Diabetes & Endocrinology | Patients from DECLARE‐TIMI‐58 trial, with creatinine clearance of at least 60 mL/min were randomly assigned to 10 mg dapagliflozin or placebo. A sustained 40% decline in eGFR to <60 mL/min per 1.73 m2, ESKD, and or death from renal or cardiovascular causes were assessed. | Dapagliflozin significantly improved the cardiorenal outcome, revealing 46% reduction in a sustained decline in eGFR and a lower risk of ESKD or renal death. |
| Cahn et al. (2019) | Efficacy and Safety of Dapagliflozin in the Elderly: Analysis From the DECLARE– TIMI 58 Study | Diabetes Care | Participants from DECLARE‐TIMI 58 trial within age subgroups were assessed for the treatment effect with dapagliflozin and age‐based treatment interaction. | Dapagliflozin reduced the composite of CV death or HHF consistently by 12% in age‐group <65, by 23% in those ≥65 to <75 years of age, and by 6% in age group ≥75 years. There was no heterogeneity in the relative risk reduction for the secondary pre‐specified cardiorenal outcome (18% to 28%) in the different age groups, revealing a consistent efficacy and safety of dapagliflozin despite age. |
AKI, acute kidney injury; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CV, cardiovascular; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESKD, end‐stage kidney disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; hsTnT, high‐sensitivity troponin T; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus; UACR, urinary albumin‐to‐creatinine ratio.
Table 9.
Characteristics of included studies and their reports for empagliflozin
| Author | Title | Journal | Methods | Findings |
|---|---|---|---|---|
|
Anker et al. (2021) (EMPEROR‐Preserved Trial) |
Empagliflozin in Heart Failure with a Preserved Ejection Fraction | The New England Journal of Medicine |
5988 patients, men or women with NYHA class II–IV HF and a LVEF > 40%, and a NT‐proBNP level of more than 300 pg/mL or, for patients with atrial fibrillation at baseline, an NT‐proBNP level of more than 900 pg/mL were randomized in this double‐blind trial to empagliflozin 10 mg vs placebo. Outcomes evaluated was composite of CV death or HHF. |
In EMPEROR‐Preserved trial, a primary outcome event occurred in 13.8% of HFpEF patients being treated with empagliflozin, an effect was closely related with a lower risk of HF hospitalization. |
|
Packer et al. (2020) (EMPEROR‐Reduced Trial) |
Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure | The New England Journal of Medicine | 3730 patients with NYHA class II, III, or IV HF and a LVEF ≤40%, with NT‐proBNP levels of at least 1000 pg/mL in those with LVEF of 31–35% or a level of at least 2500 pg/mL in those with a LVEF of 36–40%, and a level of at least 600 pg/mL in those with a LVEF of 30% or less, were randomized to empagliflozin 10 mg or placebo, as added to recommended therapy; composite of CV death or hospitalization for worsening HF was measured. |
In EMPEROR‐Reduced trial, a primary outcome event occurred in 19.4% of HFrEF patients in the empagliflozin group. Also, the rate of the decline in the eGFR was slower with empagliflozin than placebo throughout the double‐blind treatment period |
|
Zinman et al. (2015) (EMPA‐REG OUTCOME Trial) |
Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes | The New England Journal of Medicine | 7020 patients were randomly assigned to empagliflozin 10 mg or 25 mg or placebo, and empagliflozin's effects on cardiovascular morbidity and mortality in patients with T2DM at high risk for CV events were evaluated. | When empagliflozin was added to standard therapy, death from CV causes, non‐fatal myocardial infarction, or non‐fatal stroke occurred in a considerably lower percentage (10.5%) of patients. |
| Reports from EMPEROR‐Preserved, EMPEROR‐Reduced, and EMPA‐REG OUTCOME | ||||
|
Packer et al. (2021) |
Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR‐Reduced Trial | Circulation | Participants from EMPEROR‐Reduced trial, were randomly assigned to placebo or empagliflozin 10 mg daily, in addition to their usual therapy for HF, and the effect of the drug on inpatient and outpatient events reflecting worsening HF was investigated. | In HFrEF patients, empagliflozin treatment resulted in a 30% risk reduction in inpatient and outpatient worsening HF events, an effect which was statistically significant, with fewer total (first and recurrent) hospitalizations for HF. |
| Januzzi et al. (2021) |
Prognostic Importance of NT‐proBNP and Effect of Empagliflozin in the EMPEROR‐Reduced Trial |
Journal of the American College of Cardiology | Participants from EMPEROR‐Reduced study were randomized to placebo or empagliflozin 10 mg daily, and NT‐proBNP was measured at baseline, 4 weeks, 12 weeks, 52 weeks, and 100 weeks; classified into quartiles. The role and pattern of NT‐proBNP levels in patients with HFrEF treated with SGLT2 inhibitors were investigated. |
Empagliflozin significantly lowered NT‐proBNP concentrations in HFrEF patients, particularly those at greatest risk with the highest baseline NT‐proBNP concentration. At 12 weeks, empagliflozin‐treated patients were 27% more likely than placebo‐treated individuals to have an NT‐proBNP level of <1115 pg/mL. |
| Packer et a. (2021) | Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR‐Preserved Trial | Circulation | Participants from EMPEROR‐Preserved trial were randomly assigned double‐blind treatment with empagliflozin 10 mg or placebo, in addition to usual therapy, and its effect on CV death and HHF was evaluated. |
Empagliflozin was observed to reduce the combined risk of CV death, HHF, or an emergency or urgent HF visit needing intravenous therapy by 23% in HFpEF patients, which approached statistical significance. The impact was equivalent in patients with an ejection fraction of >40% to 50% and 50% to 60%, but it was diminished at higher ejection fractions. |
| Ferreira et al. (2021) | Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure EMPEROR‐Reduced | Journal of the American College of Cardiology | In EMPEROR‐Reduced participants, the mutual influence of empagliflozin and MRAs in HFrEF was examined. | Effect of empagliflozin on reducing adverse HF and renal outcomes was not influenced by the use of MRAs. Nevertheless, treatment with empagliflozin was linked to less discontinuation of MRA therapy. |
| Packer et al. (2021) | Empagliflozin in Patients With Heart Failure, Reduced Ejection Fraction, and Volume Overload EMPEROR‐Reduced Trial | Journal of the American College of Cardiology | In participants of EMPEROR‐Reduced trial, particularly those with volume overload, the baseline use of diuretics was assessed. | The study's findings did not indicate that diuresis played a role in mediating the physiological changes or therapeutic advantages of SGLT2 inhibitors on the development of HF in patients with reduced ejection fraction. |
| Ceriello et al. (2020) | Empagliflozin reduced long‐term HbA1c variability and cardiovascular death: insights from the EMPA‐REG OUTCOME trial | Cardiovascular Diabetology | In EMPA‐REG OUTCOME trial participants, the association between CV death and HbA1c variability was examined in placebo and empagliflozin arms. | Empagliflozin decreased HbA1c variability, and high HbA1c variability levels were linked to a higher risk of CV death. The decrease in HbA1c variability did not seem to be a mediator of empagliflozin's reduction in cardiovascular mortality. |
| Verma et al. (2020) | Empagliflozin reduces the risk of mortality and hospitalization for heart failure across Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes categories: Post hoc analysis of the EMPA‐REG OUTCOME trial | Diabetes, Obesity and Metabolism | Patients from EMPA‐REG OUTCOME trial were stratified into low‐intermediate, high, very‐high risk categories using baseline TRS‐HFDM, to evaluate if empagliflozin reduced the risk of CV outcomes across these categories. | Increased mortality and HHF risk were linked to greater TRS‐HFDM levels. All TRS‐HFDM category CV outcomes were decreased by empagliflozin. |
| Fitchett et al. (2019) | Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA‐REG OUTCOME trial | European Heart Journal | Participants from EMPA‐REG OUTCOME trial were treated with empagliflozin 10 or 25 mg, and the relationship between hypoglycaemia and cardiovascular outcomes was investigated. | A higher risk of HHF and MI was linked to hypoglycaemia. With empagliflozin, there was no elevated risk of hypoglycaemia, nor did incident hypoglycaemia diminish its cardioprotective benefits. |
| Inzucchi et al. (2019) | Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose‐lowering therapy? | Diabetes, Obesity and Metabolism | In participants of EMPA‐REG OUTCOME, the effect of background diabetes therapy on the risk of CV death, HHF, and progression of CKD was investigated with empagliflozin treatment. | Empagliflozin along with other glucose‐lowering agents, consistently lowered risks of adverse CV events and mortality, but with more benefit when not taken with metformin for chronic kidney disease progression. |
| Wanner et al. (2018) | Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA‐REG OUTCOME Trial | Journal of The American Society of Nephrology | Participants from EMPA‐REG OUTCOME received empagliflozin, placebo, or standard of care, and eGFR slopes were calculated. | Across all time periods, empagliflozin was associated with consistent shifts in individual eGFR slopes. The haemodynamic effects of empagliflozin, which are related to a decrease in intraglomerular pressure, may contribute to long‐term kidney function preservation. |
| Wanner et al. (2018) | Empagliflozin and Clinical Outcomes in Patients With Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease | Circulation | Participants from EMPA‐REG OUTCOME, randomized to empagliflozin 10 mg, 25 mg, or placebo along with the standard of care, were examined for outcomes of CV death, HHF, all‐cause hospitalization, and all‐cause mortality. | In T2DM patients with established cardiovascular disease, and chronic renal disease, empagliflozin improved clinical outcomes and decreased mortality; it decreased the risk of CV death by 29%, risk of HHF by 39%, and risk of all‐cause hospitalization by 19%. |
| Fitchett et al. (2018) | Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA‐REGOUTCOME trial | European Heart Journal | In this EMPA‐REG OUTCOME analysis, participants were investigated for the benefits of empagliflozin across the spectrum of HF risk. | Both in patients with low and high HF risk, empagliflozin consistently improved HF outcomes (by 29%). |
CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRAs, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus; TRS‐HFDM, Thrombolysis In Myocardial Infarction (TIMI) Risk Score for Heart Failure in Diabetes.
Table 10.
Characteristics of included studies and their reports for ertugliflozin
| Author | Title | Journal | Methods | Findings |
|---|---|---|---|---|
|
Cannon et al. (2020) (VERTIS‐CV Trial) |
Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes | The New England Journal of Medicine | 8246 T2DM patients with established ASCVD underwent randomization to ertugliflozin or placebo, followed for a mean of 3.5 years. | With regard to MACE, ertugliflozin was non‐inferior to placebo among type 2 diabetic patients with ASCVD. |
| Reports from VERTIS‐CV | ||||
| Dagogo‐Jack et al. (2022) | Cardiorenal outcomes with ertugliflozin assessed according to baseline glucose‐lowering agent: An analysis from VERTIS CV | Diabetes, Obesity and Metabolism | In this analysis from VERTIS‐CV trial, time to the first event of CV death, MI, or stroke (MACE), and other CV outcomes were assessed. | Ertugliflozin's effects on cardiorenal outcomes were consistent across patient subgroups stratified by baseline glucose‐lowering agent. The extent of HbA1c, body weight, SBP, eGFR, and UACR reductions with ertugliflozin did not differ significantly by baseline glucose‐lowering drug class. |
| Cherney et al. (2021) | Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomized VERTIS CV trial | Diabetologia | In VERTIS‐CV participants, who were randomized to receive ertugliflozin or matching placebo, added on to existing treatment, doubling of serum creatinine was replaced with sustained 40% decrease from baseline eGFR, and the effect on UACR and eGFR were assessed. | Ertugliflozin, when administered in addition to standard‐of‐care drugs, was related to a lower chance of a sustained 40% reduction in eGFR, reduced albuminuria, and eGFR preservation over time in patients with T2DM and established ASCVD. |
| Cosentino et al. (2020) | Efficacy of Ertugliflozin on Heart Failure–Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease Results of the VERTIS CV Trial | Circulation | Patients from VERTIS‐CV trial, both with LVEF ≤45% and LVEF >45%, were randomly assigned to ertugliflozin or placebo. Primary outcome assessed was the time to first MACE. |
Ertugliflozin reduced total events of HHF/CV death by 17% and total HHF events by 30%. Ertugliflozin reduced the incidence of first and total HHF, as well as total HHF/CV death, in individuals with T2DM, providing additional support for the use of SGLT2 inhibitors in the primary and secondary prevention of HHF. |
ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HHF, hospitalization for heart failure; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MI, myocardial infarction; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus; UACR, urinary albumin‐to‐creatinine ratio.
Table 11.
Characteristics of included studies and their reports for sotagliflozin
| Author | Title | Journal | Methods | Findings |
|---|---|---|---|---|
|
Bhatt et al. (2020) (SOLOIST‐WHF Trial) |
Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure | The New England Journal of Medicine | 1222 patients with T2DM who were recently hospitalized for worsening HF, were randomly assigned to sotagliflozin or placebo. | The SGLT2/SGLT1 inhibitor sotagliflozin significantly reduced the total number of CV deaths, hospitalizations, and urgent visits for HF when compared with placebo. This result was seen in all pre‐specified subgroups, including those with reduced or mid‐range (<50%) or preserved (≥50%) LVEF and those receiving the first dose before or after discharge. |
|
Bhatt et al. (2020) (SCORED Trial) |
Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease | The New England Journal of Medicine | 10 584 patients with T2DM, CKD, and risks for CV disease were randomly assigned sotagliflozin or placebo. The trial's primary endpoint was altered to a composite of the total number of deaths from CV causes, HHF, and urgent HF visits. | Sotagliflozin was associated with adverse events but resulted in a lower risk of the composite of deaths from CV causes, HHF, and urgent visits for HF in patients with diabetes and CKD, with or without albuminuria, compared with placebo (23% of patients in the sotagliflozin group). |
| Reports from SOLOIST‐WHF and SCORED | ||||
| Szarek et al. (2021) | Effect of Sotagliflozin on Total Hospitalizations in Patients With Type 2 Diabetes and Worsening Heart Failure A Randomized Trial | Annals of Internal Medicine | Participants from SOLOIST‐WHF trial were randomly assigned either sotagliflozin or matching placebo, stratified by LVEF (<50% vs. ≥50%). Alternative efficacy endpoints such as days alive and out of the hospital and percent DAOH were predetermined. | Sotagliflozin raised DAOH by 3% more than with placebo, a metric that could offer an additional patient‐centred outcome to reflect the full burden of HF. |
CKD, chronic kidney disease; CV, cardiovascular; DAOH, days alive and out of the hospital; HF, heart failure; HHF, hospitalization for heart failure; LVEF, left ventricular ejection fraction; SGLT1, sodium‐glucose cotransporter 1; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus.
Altogether, 83 878 patients were included in this review, from the 12 major RCTs that were assessed, and all the studies used a placebo control. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Among the included studies, two RCTs, with six respective reports 35 , 36 , 37 , 38 , 39 , 40 investigated canagliflozin, four RCTs with 13 derived reports 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 investigated dapagliflozin, three RCTs with 12 respective reports 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 studied the effects of empagliflozin, one RCT and its three respective reports 65 , 66 , 67 assessed ertugliflozin's effects, and finally, two RCTs with one added report 68 investigated the dual inhibitor sotagliflozin.
3.2.1. Canagliflozin
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) 23 and The Canagliflozin Cardiovascular Assessment Study (CANVAS) 24 RCTs indicated that canagliflozin reduced the risk of cardiovascular death, myocardial infarction, stroke, and HF hospitalization (Tables 2 and 7 ).
3.2.2. Dapagliflozin
In patients with chronic HF and reduced LVEF, RCTs investigating dapagliflozin, namely, Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure (DELIVER), 25 Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA‐CKD), 26 Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA‐HF), 27 and Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE‐TIMI 58), 28 indicated a lower risk of HF hospitalization and death from cardiovascular causes in HF across the ejection fraction range (HFrEF, HFmrEF, and HFpEF), as well as a lower risk of a sustained 50% decline in eGFR, end‐stage kidney disease, and death from renal causes, in CKD patients (Tables 3 and 8 ).
3.2.3. Empagliflozin
Empagliflozin Outcome Trial in Patients with Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR‐Preserved), 29 Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR‐Reduced), 30 and Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA‐REG OUTCOME), 31 indicated that the combined risk of cardiovascular death or hospitalization was decreased with empagliflozin in both HF with preserved and reduced ejection fraction (Tables 4 and 9 ).
3.2.4. Ertugliflozin
Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial (VERTIS‐CV), 32 which investigated the effects of ertugliflozin, indicated that MACE did not differ significantly in patients with atherosclerotic cardiovascular disease, as well as cardiorenal outcomes in these patients and in those with diabetes mellitus were unaffected by baseline glucose‐lowering agents (Tables 5 and 10 ).
3.2.5. Sotagliflozin
The dual SGLT inhibitor sotagliflozin was studied in the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST‐WHF) 33 and Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) 34 trials, which indicated that it reduced the risk of death from cardiovascular causes, HF hospitalizations, and urgent hospital visits for worsening heart failure (Tables 6 and 11 ).
3.3. Risk of bias assessment of the included randomized controlled trials
The included studies were sufficiently well reported to allow a full assessment of risk of bias. All 12 studies provided appropriate information on randomization methods and process, whereby in four of them, 26 , 27 , 29 , 34 the randomization was described in scrutiny to have been performed with a permuted block design and stratified by geographic region, criteria for HF, diabetes status, estimated glomerular filtration rate (eGFR), urinary albumin‐to‐creatinine ratio, or use of balanced blocks of 1:1 ratio of the regimens involved. The remaining eight trials were judged as low risk of bias in the random sequence generation domain as well.
Regarding the allocation concealment domain, in seven trials 24 , 26 , 27 , 28 , 29 , 32 , 34 central randomization through the use of an interactive voice‐response or Web‐response system by the investigators, to determine trial‐group assignments was specified, and the rest, were judged as low‐risk of bias, with the exception of two trials 23 , 31 that were judged as unclear risk of bias.
Blinding of participants and personnel (performance bias) was judged as low risk of bias for nine trials due to the double‐blind nature, whereas three trials 30 , 31 , 32 were judged as unclear risk of bias, because it was stated that following randomization, all relevant therapies could be started or modified based on each patient's needs, at health care provider's clinical discretion.
The blinding of outcome assessment domain (detection bias) was judged as low risk of bias in 10 trials, as backed up by the information specified in the protocol that all patients adhered to the visit schedule, participants and all trial personnel were unaware of the trial‐group assignments, as well as outcomes were adjudicated in a blinded manner according to pre‐specified definitions by the clinical‐events committees. Two trials 24 , 31 were regarded as unclear risk of bias in this domain.
Attrition bias judged as high risk for one trial, 33 as trial enrolment was closed early subsequent to loss of funding from the sponsor, which resulted in a substantial reduction in power to test the original primary endpoint; also, the intended adjudication of events could not be completed. Attrition bias was judged as unclear risk of bias for three trials. 23 , 28 , 31 There was an increased risk of lower limb amputation was identified with canagliflozin. Investigators temporarily interrupted the assigned treatment in patients with any active condition would lead to amputation. This domain for the remaining studies was judged as low risk of bias, because even if there were missing data, the protocol specified that sensitivity analyses were performed with the use of an intention‐to‐treat approach.
All 12 other trials were judged as low risk of bias for the selective reporting domain, owing to the fact that any adverse events were clearly reported in the study, whether they resulted in the discontinuation of the intervention, or were simply adverse events of interest (volume depletion symptoms, major hypoglycaemia, bone fractures, amputations, renal events, and potential diabetic ketoacidosis).
Other bias for all 12 studies was judged as unclear.
Quality assessment items are presented in Figure 2 .
Figure 2.

Risk of bias summary. Review authors' judgements about each risk of bias item for each of the 12 included RCTs. Created with Revman Software (Review Manager 5.4.1), upon completion of quality assessment for each study.
3.4. Meta‐analysis results: Effect of sodium‐glucose cotransporter 2 inhibitors on atrial fibrillation in heart failure patients
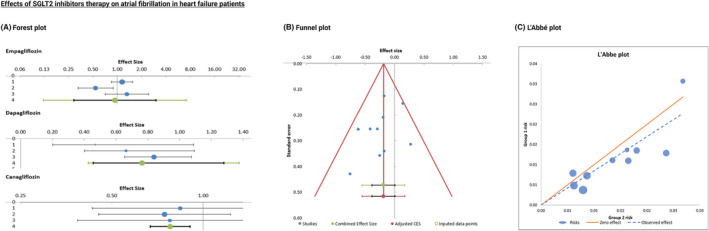
Of the included trials in the systematic review, 10 studies were chosen to undergo meta‐analysis specifically aimed at the pooled effects of SGLT2 inhibitors therapy on AF (as one of the serious adverse events reported) in HF patients. Figure 3 illustrates the forest plot of the meta‐analysis, providing the combined effect size and heterogeneity results. In Figure 4 and Figure 5 , results from the publication bias analysis, including funnel plot and L'Abbé plot results, are depicted.
Figure 3.
(A) Forest plot of placebo‐controlled randomized trials examining the pooled effects of SGLT2 inhibitors therapy on AF occurrence in heart failure patients. The meta‐analytic results (lines 4) consist of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. CI is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval. (B) Funnel plot of placebo‐controlled randomized trials examining the effects of SGLT2 inhibitors therapy on AF in heart failure patients, depicting effect sizes against their standard errors. (C) L'Abbé plot showing the AF event rate in the intervention group (SGLT2 inhibitors) against the AF event rate in the placebo group.
Figure 4.
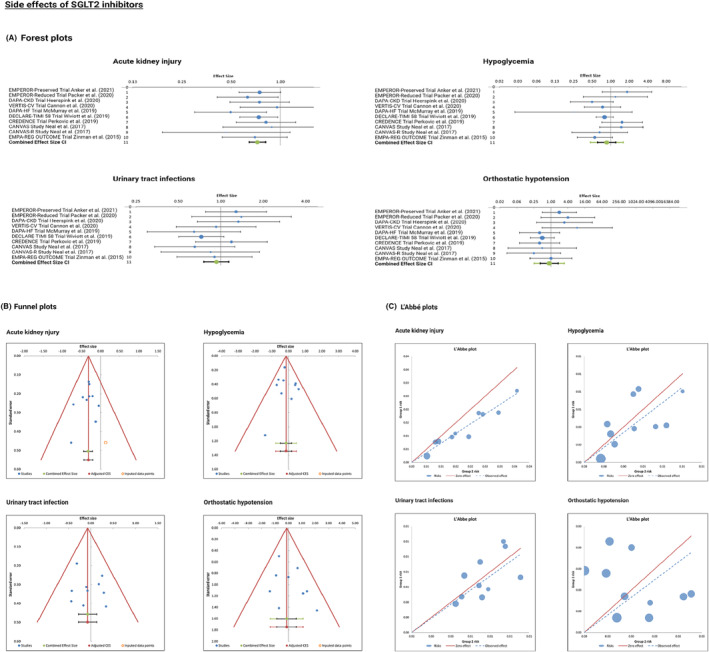
(A) Forest plot of placebo‐controlled randomized trials examining the pooled effects of SGLT2 inhibitors‐related common side effects. The meta‐analytic results (lines 11) consist of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. Confidence interval is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval. (B) Funnel plot of placebo‐controlled randomized trials examining the side effects of SGLT2 inhibitors, depicting effect sizes against their standard errors. (C) L'Abbé plot showing the side effects in the intervention group (SGLT2 inhibitors) against the side effects in the placebo group.
Figure 5.

Forest plot of placebo‐controlled randomized trials comparing the pooled effects of SGLT2 inhibitors, GLP‐1 agonists, and DPP‐4 inhibitors on HHF. The meta‐analytic results (lines 5) consist of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. Confidence interval is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval.
The included studies were entered in the following order, according to the year of publication, from newest to oldest, with the respective results for each individual study: EMPEROR‐Preserved trial, EMPEROR‐Reduced trial, DAPA‐CKD trial, VERTIS‐CV trial, DAPA‐HF trial, DECLARE‐TIMI 58 trial, CREDENCE trial, CANVAS study, CANVAS‐R study, and EMPA‐REG OUTCOME trial. The respective odds ratios (OR), CI, weight, heterogeneity, and combined effect size results were reported in Appendix S5 .
Overall effect (weighted average effect) is <1, but the range of 95% CI of the combined effect size overlaps with the value of 1, indicating that the meta‐analytic effect is not statistically significant (no benefit or harm in terms of SGLT2 inhibitors therapy and AF occurrence; no significant difference between the intervention and comparison group) (Figure 3 ). Furthermore, heterogeneity was low; thus, there was nothing to be explored in a subgroup or moderator analysis. P value was greater than 0.05, suggesting all the studies on the left side of the plot were homogenous (no heterogeneity).
We performed quantitative analysis using Egger's linear regression test to assess the funnel plot for asymmetry evidence, as measured by the intercept from regression of standard normal deviates against precision (the intercept did not differ significantly from zero). Despite the apparent asymmetry, Egger's test for publication bias was not statistically significant (P = 0.268). The L'Abbé plot displayed in Figure 3 was useful in investigating the heterogeneity of effect estimates within this meta‐analysis.
3.5. Meta‐analysis results: Common side effects of the sodium‐glucose cotransporter 2 inhibitors
The included studies for meta‐analysing the common side effects with SGLT2 inhibitors were as follows: EMPEROR‐Preserved trial, EMPEROR‐Reduced trial, DAPA‐CKD trial, VERTIS‐CV trial, DAPA‐HF trial, DECLARE‐TIMI 58 trial, CREDENCE trial, CANVAS study, CANVAS‐R study, and EMPA‐REG OUTCOME trial. The respective odds ratio (OR), CI, weight, heterogeneity, and combined effect size results were reported in Appendix S6 .
3.5.1. Acute kidney injury
In three of the studies (to the left side of 1), it was indicated that patients treated with empagliflozin or dapagliflozin were less likely to suffer from AKI as an adverse event, with statistically significant findings owing to both the individual effect sizes and the combined effect size not crossing the null value and that it was not included in the 95% CIs ranges.
3.5.2. Hypoglycaemia
The results were not statistically significant as the value of 1 was included in the 95% CIs range of the individual studies as well as in the combined effect size CI (Figure 4A ).
3.5.3. Urinary tract infections
Findings also were not statistically significant, as the line of no effect was crossed by all individual studies, including their 95% Cis range.
3.5.4. Orthostatic hypotension
One study, EMPA‐REG OUTCOME, fell exactly on the line of no effect, indicating that results were insignificant with regard to orthostatic hypotension.
Intercept values of Egger regression regarding the publication bias analysis can be found in Appendix S6 .
3.6. Meta‐analysis results: Comparison of the effects of the sodium‐glucose cotransporter 2 inhibitors, glucagon‐like‐peptide‐1 agonists, and dipeptidyl peptidase‐4 inhibitors on hospitalization for heart failure, cardiovascular death, and major adverse cardiovascular events
The studies were compared in the context of HHF, cardiovascular death, and MACE occurrences. For each of the three endpoints, four trials were selected based on data availability in the clinical trial registries. VERTIS‐CV, DECLARE‐TIMI 58, CREDENCE, and EMPA‐REG OUTCOME trials for SGLT2 inhibitors' effects, AMPLITUDE‐O, REWIND, EXSCEL, and SUSTAIN‐6 trials for GLP‐1 agonists' effects, as well as CARMELINA, OMNEON, TECOS, SAVOUR‐TIMI 53 trials for DPP‐4 inhibitors' effects were meta‐analysed for the chosen outcomes. The respective odds ratios (OR), CI, weight, heterogeneity, and combined effect size results were reported in Appendix S7 – S9 .
3.6.1. Hospitalization for heart failure
The studies investigating the effect of SGLT2 inhibitors on HHF indicated that patients treated with these SGLT2 inhibitors were less likely to experience HHF (left side of 1). In one study investigating the effect of GLP‐1, namely, SUSTAIN‐6 trial (right side of 1), placebo group was at risk of experiencing HHF as compared with treatment group. For the rest of the studies for GLP‐1 agonists, the value of 1 (null effect) was included in the 95% CIs range of the individual studies (Figure 5 ), hence statistically insignificant. Similar findings were observed with the analysis of studies investigating DPP‐4 inhibitors.
3.6.2. Cardiovascular death
The studies investigating the effect of SGLT2 inhibitors indicated that patients treated with these SGLT2 inhibitors were less likely to die from cardiac reasons (cardiovascular death) (left side of 1). However, the value of 1 (null effect) was included in the 95% CIs range of the individual studies. Results were similar for GLP‐1 agonists studies, but the combined effect size CI did not cross the line of no effect, indicating statistically significant pooled results (Figure 6 ). No significant results were observed for the studies investigating DPP‐4 inhibitors.
Figure 6.
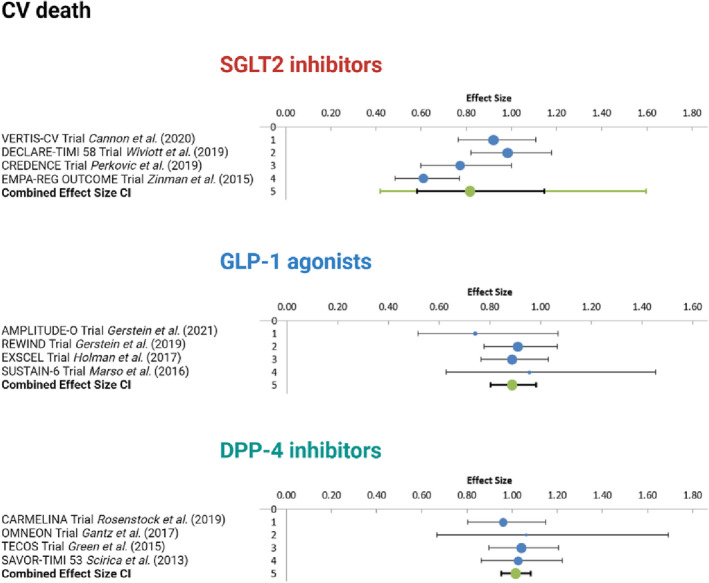
Forest plot of placebo‐controlled randomized trials comparing the pooled effects of SGLT2 inhibitors, GLP‐1 agonists, and DPP‐4 inhibitors on cardiovascular death. The meta‐analytic results (lines 5) consist of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. Confidence interval is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval.
3.6.3. Major adverse cardiovascular events
Three individual studies, as well as the combined effect size CI, indicated that patients treated with these SGLT2 inhibitors were less likely to experience major adverse cardiovascular events (left side of 1), but the value of 1 was included in the 95% CIs range of the individual studies; therefore, results were insignificant. Three studies, AMPLITUDE‐O trial, REWIND trial, and SUSTAIN‐6 trial, indicated that patients treated with GLP‐1 agonists were less likely to have MACE occurrence (to the left side of 1), with the value of 1 not included in the 95% CIs range, but the combined effect size CI did cross the line of no effect (Figure 7 ). Regarding the DPP‐4 inhibitors studies, one study, namely, CARMELINA trial, the range of 95% CI crossed the value 1, which means that the difference in effects was not significant.
Figure 7.

Forest plot of placebo‐controlled randomized trials comparing the pooled effects of SGLT2 inhibitors, GLP‐1 agonists, and DPP‐4 inhibitors on MACE. The meta‐analytic results (lines 5) consist of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. Confidence interval is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval.
Intercept values of Egger regression regarding the publication bias analysis alongside the Funnel plots and L'Abbé plots (Figure S1) can be found in Appendix S10 .
3.7. Meta‐analysis results: Comparison of the effects of the sodium‐glucose cotransporter 2 inhibitors, glucagon‐like‐peptide‐1 agonists, and dipeptidyl peptidase‐4 inhibitors on end‐stage renal disease
The included studies were the following order, with the respective results for each individual study: EMPEROR‐Preserved trial, EMPEROR‐Reduced trial, VERTIS‐CV trial, DAPA‐CKD trial, DAPA‐HF trial, DECLARE‐TIMI 58 trial, and CREDENCE trial for SGLT2 inhibitors, displayed on the forest plot (Figure 8 ), as well as the REWIND trial (OR = 0.83, 95% CI: 0.49–1.42, weight = 21.81%) for GLP‐1 agonists, and CARMELINA trial (OR = 0.84, 95% CI: 0.54–1.30, weight = 32.75%) for DPP‐4 inhibitors. Notably, heterogeneity was low, and the combined effect size for all the included studies was: OR = 0.76, 95% CI: 0.64–0.91, PI: 0.64–0.91, without crossing the line of no effect overall. The highest weighted average was in the GLP‐1 agonist trial (CARMELINA), followed by SGLT2 inhibitors (DAPA‐CKD), and the DPP‐4 inhibitor trial (REWIND).
Figure 8.
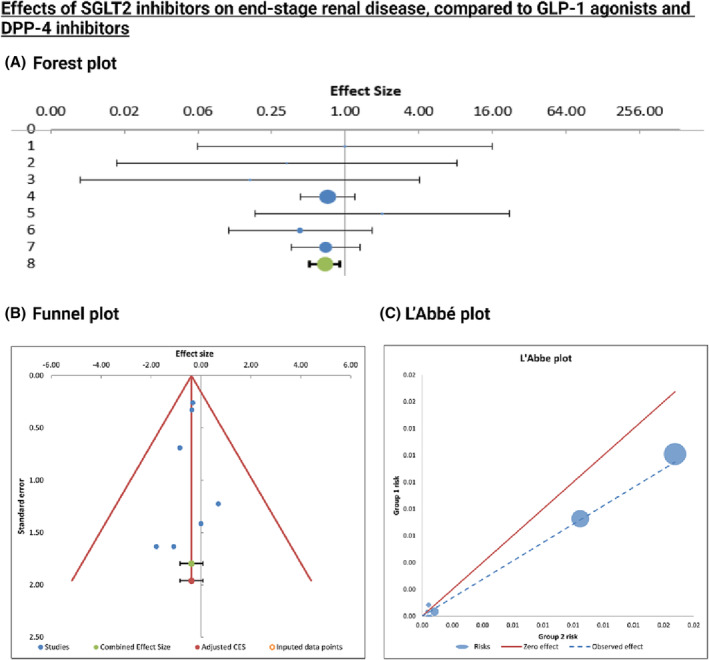
(A) Forest plot of placebo‐controlled randomized trials examining the pooled effects of SGLT2 inhibitors effects on end‐stage renal disease. The meta‐analytic result (line 8) consists of two intervals, both around the same bullet, which represent the weighted average effect or the combined effect size. Confidence interval is represented by the smaller, black interval, whereas prediction interval is represented by the larger green interval. (B) Funnel plot of placebo‐controlled randomized trials examining the effects of SGLT2 inhibitors on end‐stage renal disease, depicting effect sizes against their standard errors. (C) L'Abbé plot showing the side effects in the intervention group (SGLT2 inhibitors) against the side effects in the placebo group.
Intercept values of Egger regression regarding the publication bias analysis can be found in Appendix S11 .
4. Discussion
The RCTs investigating the effects of the SGLT2 inhibitors found robust results on significant reductions in three‐point MACE (MACE‐3), HHF, as well as renal function in patients with HF across the range of phenotypes according to LVEF (HFrEF, HFmrEF, and HFpEF). Additionally, a meta‐analytic effect of AF as one of the adverse events in HF patients following SGLT2 inhibitor treatment was calculated.
The gap in evidence towards gliflozins' effects on HFpEF and HFmrEF was addressed recently in the DELIVER trial, whereby Solomon et al. reported significant results about the effectiveness of dapagliflozin in reducing the combined risk of worsening HF or cardiovascular death in patients with HF and preserved (HFpEF) or mildly reduced ejection fraction (HmrEF). In patients with HF and a preserved ejection fraction, empagliflozin treatment decreased the combined risk of cardiovascular death or hospitalization for HF in the EMPEROR‐Preserved trial. Main findings from SOLOIST‐WHF trial were in the function of reduced risk of death from cardiovascular causes, HF hospitalization, and urgent hospital visit for HF with the dual (SGLT1/SGLT2) inhibitor sotagliflozin. The EMPEROR‐Reduced trial deduced that the combined risk for cardiovascular death or HF hospitalization was reduced by 25% following treatment with empagliflozin. Dapagliflozin treatment in the DAPA‐HF trial also found that patients with chronic HF and reduced LVEF had lower mortality and HF‐related adverse events by 25%. In the EMPA‐REG OUTCOME trial, MACE‐3 rate was significantly reduced, as well as the risk of cardiovascular death was reduced (by 38%) with empagliflozin once‐daily therapy. In the DAPA‐CKD trial, dapagliflozin lowered the risk of HF hospitalization and death from cardiovascular causes. Likewise, sotagliflozin therapy in the SCORED trial found that there was a lower risk of the total number of cardiovascular fatalities, hospitalizations for HF, and urgent hospital visits for HF. Additionally, the CANVAS Program and CREDENCE trial indicated that canagliflozin treatment significantly lowered the rates of death from cardiovascular causes, non‐fatal myocardial infarction, or non‐fatal stroke, as well as HF hospitalization. DECLARE‐TIMI 58 trial revealed that dapagliflozin had no effect on the rate of MACE in patients with T2DM and high cardiovascular risk. Curiously, the VERTIS‐CV trial found that, with ertugliflozin, results did not reach significance in patients with atherosclerotic cardiovascular disease, with rates of MACE of approximately 4% per year. Evidence from these trials has clearly illustrated a consistent effect on the combined endpoints.
AF is frequent in HF with preserved ejection fraction (HFpEF) patients and is thus associated with poor prognosis. 14 Bearing that in mind, AF was our selected outcome of interest to assess a pooled effect size, which resulted in no statistically significant difference between the intervention and placebo groups in AF occurrence, with no benefit or harm in terms of SGLT2 inhibitors therapy and AF as an adverse event. Nevertheless, a study by Butt et al. suggests that SGLT2 inhibition, particularly with dapagliflozin, led to symptom improvement in HF patients regardless of AF status, but it did not effectuate a reduction in the risk of new‐onset AF. 69 A further insight from EMPEROR‐Preserved suggested that empagliflozin has been associated with a reduction in serious HF events and slower deterioration in glomerular function in both patients with and without AF. 70 Interestingly, a recent experimental study found that dapagliflozin, as opposed to empagliflozin, had favourable effects on atrial electrophysiology resulting in the prevention of AF through a putative anti‐arrhythmic action of the drug.
Though there are established therapies directed towards diminishing the neurohormonal overactivation in patients with HF with reduced ejection fraction, the therapeutic choices for individuals with HF with a preserved ejection fraction are finite. On this note, the most tantalizing findings with respect to changing the paradigm in clinical practice were those regarding HF with preserved ejection fraction (HFpEF) in the EMPEROR‐Preserved trial and the DELIVER trial. While in DAPA‐HF dapagliflozin reduced the risk of worsening HF or cardiovascular death among HF patients with a LVEF of 40% or less, the DELIVER trial further demonstrated extended impactful results to patients with HF and a LVEF of more than 40%, similarly to the EMPEROR‐Preserved trial findings with empagliflozin, thus, throughout the full range of ejection fraction. 25 EMPEROR‐Preserved trial also enrolled patients within the mildly reduced ejection fraction range and was primarily a HF trial. In HFpEF patients, SGLT2 inhibition with empagliflozin resulted in a 21% relative risk reduction in the composite of cardiovascular death or hospitalization for HF. This effect was primarily attributable to 29% lower risk of hospitalization for HF with this intervention. 29 All pre‐specified subgroups, including individuals with or without diabetes, typically showed consistent effects on the incidence of primary outcome events. 42 , 45 , 46 , 47 , 48 Empagliflozin also resulted in a longer time to first HF hospitalization and a decrease in the total number of HF hospitalizations. Importantly, the lack of significant reduction in cardiovascular death with empagliflozin intervention in HFpEF patients in the EMPEROR‐Preserved trial may arguably be the case due to the rate of cardiovascular death in these patients being relatively low because they have a preserved LVEF, making it difficult to project a substantial effect, as many of the deaths in HFpEF patients are driven by non‐cardiovascular underlying factors.
It is noteworthy to mention that EMPEROR‐Preserved maintained the overall type I error rate by assessing the two key secondary outcomes (total – first and recurrent HHF and the slope of the change in eGFR) in a pre‐specified stepwise, hierarchical manner, provided that the between‐group difference in results for the primary outcome was substantial. 29
When compared with the results of the EMPEROR‐Reduced trial, the empagliflozin benefit pattern observed in the EMPEROR‐Preserved trial is analogous. This implies that, overall, the effects of SGLT2 inhibition on HF events do not vary significantly with the HF phenotype.
Some contrary reports argue that, regarding EMPEROR‐Preserved trial, patients with HF and a preserved ejection fraction (HFpEF) may have benefited from treatment, either at the time of randomization or a post‐randomization HF event, but these analyses had a small number of events and a vast amount of missing data. Furthermore, larger‐scale studies with the other medications used in the guideline‐directed medical therapy for HF, such as angiotensin receptor blockers, mineralocorticoid receptor antagonists, and angiotensin receptor neprilysin inhibitor (ARNI), suggested that any benefit may have been preferentially seen in patients with an LVEF of 40% to 49% (mid‐range ejection fraction); however, these patients frequently exhibit clinical characteristics more comparable to those of patients with HFrEF than with those of patients with HFpEF. Additionally, the DAPA‐HF trial demonstrated a statistically significant 25% reduction in HF hospitalization due to decompensation and cardiovascular death or all‐cause mortality, similarly to the EMPEROR‐Reduced trial. 27 Notwithstanding the presence or absence of diabetes, patients with HF and a reduced ejection fraction being treated with dapagliflozin had a lower risk of worsening HF or death from cardiovascular causes as well as better symptom scores compared with placebo. These benefits were demonstrated in all HFrEF patients, irrespective of diabetes status. 27
With respect to safety considerations, it should be noted that the EMPEROR‐Reduced trial in fact, listed the eGFR slope as one of the hierarchical endpoints; there was an initial drop in eGFR and a later stabilization, with a beneficial effect overall. 30 The same was seen in DAPA‐HF, even in patients without diabetes. The rates of AKI did not increase with SGLT2 inhibitor use in HFrEF. Indeed, the renal safety outcomes in DAPA‐HF were fewer in the dapagliflozin arm than in the placebo arm. In the EMPEROR‐Reduced trial, the renal function exclusion criterion was an eGFR below 20 mL/min. Whereas for DAPA‐HF and SOLOIST‐WHF trials, the criterion was an eGFR below 30 mL/min. 27 , 33 Data from DAPA‐HF also revealed that very few cases of diabetic ketoacidosis occurred in patients with T2DM, none in those without a history of type 2 diabetes, and a 0.2% rate of major hypoglycaemia. 27 In addition, when HbA1c was monitored over time in these trials, dapagliflozin had no meaningful blood glucose‐lowering effect in patients without diabetes. Similar results were seen in the EMPEROR‐Reduced trial in individuals with HFrEF; there were very few instances of hypoglycaemic events and no occurrences of diabetic ketoacidosis with empagliflozin therapy. 30 Their pharmacological properties render the SGLT2 inhibitors rather convenient to prescribe with a single dose, without a need for titration, and with no need for adjustment of other diuretic medications empirically. Nevertheless, it is well known that among the most vulnerable patients to treat with any pharmacological agents, including SGLT2 inhibitors, are CKD patients, who frequently require dose adjustments and in whom the cardio‐renal efficacy of drugs is inevitably compromised. Accordingly, preclinical studies have led to the impactful revelation that low‐dose SGLT2 inhibitors yield effectiveness in tackling cardiac and renal fibrosis in animal models at a level that is comparable to the gold‐standard medication. 71 This effect is mainly attributable to the manipulation of the expression of genes related to endothelial function and oxidative stress in the kidneys, explaining the possible tubuloglomerular feedback‐independent mechanisms of such low‐dose treatment. 71 Importantly, the possibility of treating the CKD animal models with a low dose SGLT2 inhibition opens up a further line of investigation in clinical studies; this holds clinical significance in providing a novel therapeutic modality in patients unable to use the already‐existing benefits of SGLT2 inhibitors.
It is worth noting that differences in effects of different SGLT2 inhibitors have been reported, mostly attributed to tissue selectivity of these pharmacological agents for SGLT2 and SGLT1. This hypothesis has been reinforced by the findings of a recent preclinical study examining renal ischaemia–reperfusion effects on rat models, which suggested that off‐target effects as a result of higher tissue selectivity for SGLT2 over SGLT1 with some of these pharmacological agents translate into differences in terms of AKI as an adverse outcome. 72 Protection against AKI may be a result of off‐target effects of these, and not due to a class effect. 72
Although these RCTs were well‐designed and provided a large sample size, some of them were still characterized by certain limitations. For example, SOLOIST‐WHF trial faced a loss of funding from the sponsor, which led to the trial being stopped before enrolment of the initially planned sample size. Although the trial revealed a favourable effect in terms of the intended primary endpoint of the first event of either death from cardiovascular causes or HF hospitalization, its earlier‐than‐anticipated termination compromised the statistical power to assess the secondary endpoints, such as death from cardiovascular causes. 33 Due to the trial's early discontinuation and the limited sample size of this subgroup, it was challenging to reach any conclusive results in this regard, allowing space for further research. Also, DAPA‐HF relied on specific inclusion and exclusion criteria, which may have limited the generalizability of the main findings. Along with that, it should be emphasized that the inclusion criteria for the very first few trials, namely, EMPA‐REG OUTCOME, CANVAS, and DECLARE‐TIMI 58, differed in comparison to the more recent trials, in the context of eligible participants being patients with a history of symptomatic atherosclerotic cardiovascular disease or those older than 50 years of age with two or more risk factors for cardiovascular disease, including a history of diabetes for at least 10 years, or with already established atherosclerotic cardiovascular disease (defined as clinically evident ischaemic heart disease, ischaemic cerebrovascular disease, or peripheral artery disease). 24 , 28 , 31 Recent trials, however, have gravitated towards including not only patients with established cardiovascular disease with focus on HF events, but crucially involving primarily HF patients as well, within the phenotype ranges, and not necessarily with a diabetes mellitus diagnosis, yet yielding unforeseen results.
When merits of the three groups of drugs (SGLT2 inhibitors, GLP‐1 agonists and DPP‐4 inhibitors) in the area of HF‐related events are compared, it is not yet possible to reach firm conclusions. While only SGLT2 inhibitors decrease risk of hospitalization due to HF, on the other hand only GLP‐1 agonists significantly decrease risk of cardiovascular death. Further clinical studies are necessary to explore potential benefits of all three groups of drugs in patients with HF. In addition, it should be noted that analysing clinical studies regarding the occurrence of a specific endpoint and the benefits of these three drug groups is quite difficult because the endpoint definitions themselves differ from study to study. The same stands to reason when approaching a patient with a certain co‐morbidity, but something similar cannot be concluded from the mentioned studies, because the studies were not head‐to‐head. This all leads to the conclusion that the clinician must still approach the patient individually, know all the benefits (or side effects) of the drug, and must know very well the methodology of each clinical study (that is, the pharmacodynamics and pharmacokinetics of each pharmacological agent that belongs to this group of drugs).
Concerning external validity, it should be taken into account that this systematic review has been based on collating results from a variety of primary studies; therefore, it is also important to consider whether the evidence reported can be directly applied to the general HF population of the three different phenotypes presented, with or without diabetes. Hence, further subgroup or sensitivity analysis of the included trials in this study, particularly on the effects of SGLT2 inhibitors on HFpEF, should be conducted to allow for additional evidence to be directly applicable to the population of interest.
Similarly to the already‐mentioned positive effects of dual inhibition with sotagliflozin in acute HF patients, such clinically relevant benefits have recently been observed in patients hospitalized for acute HF as well, in whom empagliflozin initiation improved symptoms and functional status in the early post‐discharge period, regardless of the degree of symptomatic impairment at baseline. 73 These benefits were seen as early on and were consistent in those with HF with reduced but also preserved ejection fraction, an effect that very few therapies other than sotagliflozin and empagliflozin have been shown to achieve in these types of patients. This leaves room to envision that dual SGLT inhibition will likely play a prominent role among patients with CKD and HF, possibly even in the absence of T2DM, with further investigative advancements in the upcoming years.
Moreover, contemporary research indicates that the effects of SGLT2 inhibitors on improved outcomes extend to the spectrum of patients with acute decompensated HF (the EMPULSE trial), a condition that frequently coexists with renal impairment. Specifically, empagliflozin treatment in acute decompensated HF patients did not lead to an increased risk for AKI, thereby reinforcing the renoprotective effects of SGLT2 inhibitors. 73 As HFpEF has been despicably difficult to treat, with no previous single agent having proven effective enough in improving cardiovascular outcomes in this patient population, a brave attempt to make a shift in this matter was with the infamous Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial from the Americas, which was trying to prove that perhaps spironolactone treatment in HFpEF could be a long‐awaited solution. In the TOPCAT trial, spironolactone decreased all‐cause mortality in women with HF with preserved ejection fraction, but not in men. 74 A subgroup of individuals in the TOPCAT study recruited experienced substantial reduction in the primary endpoint (cardiovascular death and HF hospitalization), and a subsequent post hoc analysis revealed a significant reduction in outcomes for those with an LVEF <55%. 74 Nonetheless, the results of this trial indicated that spironolactone did not demonstrate superiority compared with placebo in terms of improving cardiovascular outcomes in HFpEF patients. Over and above that, it was related to a higher rate of hyperkalemia and renal failure. Yet the reduction in HF hospitalizations with this medication leaves space for emerging hypotheses gravitating towards the treatment of HFpEF. Hospitalizations for HF were reduced by spironolactone in LVEF below the normal range.
This study brought together the findings from the major RCTs with detailed reference to each of the five so‐far investigated drugs: empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, and sotagliflozin. The rigour of the methodology performed with the detailed and extensive search strategies allows for future reproducibility of this research.
5. Conclusions
SGLT2 inhibitors significantly improve the quality of life in HF patients. Their beneficial effects on HF, especially in left ventricular dysfunction, have made their use possible irrespective of diabetes mellitus. Other anti‐hyperglycaemic medications do not confer the same benefits as SGLT2 inhibitors in terms of reducing the risk of serious adverse renal events and HF hospitalization. Inhibiting both intestinal and renal glucose absorption, boosting GLP‐1 release, and protecting cardiac tissue by reducing glycogen accumulation are all effects of simultaneous inhibition of SGLT2 and SGLT1, which contributes to this class of drugs' remarkable safety and efficacy. SGLT2 inhibitors and GLP‐1 agonists are now recommended by cardiovascular pharmacology guidelines for the treatment of patients diagnosed with T2DM and cardiovascular disease. Therefore, their use should be prioritized based on the patient's diabetes mellitus profile and cardiovascular risk. In addition to their clinical use in HFrEF patients, SGLT2 inhibitors have demonstrated efficacy by decreasing cardiovascular death and HF hospitalizations in HFmrEF and HFpEF patients. As a result, they are now also considered as guideline‐directed medical therapy for both of these HF phenotypes. Treatment with SGLT2 inhibitors should be individualized based on the patient's diabetes mellitus profile and cardiovascular risk, as well as drug properties. The clinical significance of dual SGLT2 inhibitors is reinforced by early therapeutic implementation, increasing the likelihood of more favourable outcomes.
Conflict of interest
There are no conflicts of interest.
Supporting information
Appendix S1. Detailed search strategy on PubMed/Medline database.
Appendix S2. Detailed search strategy on Cochrane Central Register of Controlled Trials (CENTRAL) database.
Appendix S3. Search strategy from ClinicalTrials.gov.
Appendix S4. Numbers of included randomized controlled trials registered at ClinicalTrials.gov registry.
Appendix S5. Effects of SGLT2 inhibitors therapy on atrial fibrillation in heart failure patients: results from meta‐analysis.
Appendix S6. Common side effects of the SGLT2 inhibitors: results from meta‐analysis.
Appendix S7. Effects of the SGLT2 inhibitors on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S8. Effects of the GLP‐1 agonists on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S9. Effects of the DPP‐4 inhibitors on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S10. Publication bias analysis: results from meta‐analysis.
Appendix S11. Effects of SGLT2 inhibitors on end‐stage renal disease (ESRD) and publication bias analysis: results from meta‐analysis.
Aziri, B. , Begic, E. , Jankovic, S. , Mladenovic, Z. , Stanetic, B. , Kovacevic‐Preradovic, T. , Iglica, A. , and Mujakovic, A. (2023) Systematic review of sodium‐glucose cotransporter 2 inhibitors: a hopeful prospect in tackling heart failure‐related events. ESC Heart Failure, 10: 1499–1530. 10.1002/ehf2.14355.
References
- 1. Braunwald E. SGLT2 inhibitors: the statins of the 21st century. Eur Heart J. 2022; 43: 1029–1030. [DOI] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Butler J. SGLT‐2 inhibitors in heart failure: a new therapeutic avenue. Nat Med. 2019; 25: 1653–1654. [DOI] [PubMed] [Google Scholar]
- 3. Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018; 61: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunwald E. Gliflozins in the management of cardiovascular disease. N Engl J Med. 2022; 386: 2024–2034. [DOI] [PubMed] [Google Scholar]
- 5. Choi CI. Sodium‐glucose cotransporter 2 (SGLT2) inhibitors from natural products: discovery of next‐generation antihyperglycemic agents. Molecules. 2016; 21: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verma S, McMurray JJ. The serendipitous story of SGLT2 inhibitors in heart failure: new insights from DECLARE‐TIMI 58. Circulation. 2019; 139: 2537–2541. [DOI] [PubMed] [Google Scholar]
- 7. Das US, Paul A, Banerjee S. SGLT2 inhibitors in heart failure with reduced ejection fraction. Egypt Heart J. 2021; 73: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadro W, Al Turkmani M, Kadro K, Kadro MY, Rigali D, Pietro G, Hamid M, Mathew S. The effect of SGLT2 inhibitors on silent myocardial ischemia. Eur Heart J. 2020; 41 ehaa946–ehaa1446. [Google Scholar]
- 9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JG. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 11. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, De Boer RA, Farmakis D. Type 2 diabetes mellitus and heart failure: a position statement from the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 12. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016; 65: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 13. Despa S. Myocyte [Na+] i dysregulation in heart failure and diabetic cardiomyopathy. Front Physiol. 2018; 9: 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009; 119: 2516–2525. [DOI] [PubMed] [Google Scholar]
- 15. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015; 36: 3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yanai H. Acute effects of preventing heart failure by sodium‐glucose cotransporter 2 inhibitors. Cardiol Res. 2021; 12: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium‐glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci. 2016; 130: 159–169. [DOI] [PubMed] [Google Scholar]
- 18. Uthman L, Baartscheer A, Schumacher CA, Fiolet JW, Kuschma MC, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018; 9: 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009; 119: 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015; 54: 691–708. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Ropero A, Badimon JJ, Santos‐Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol. 2018; 14: 1287–1302. [DOI] [PubMed] [Google Scholar]
- 22. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016; 134: 752–772. [DOI] [PubMed] [Google Scholar]
- 23. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 24. Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 25. Solomon SD, McMurray JJ, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CS, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang C‐E, Borleffs CJW, Comin‐Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer‐Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Kerr Saraiva JF, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Nguyen Pham V, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM, DELIVER Trial Committees and Investigators . Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022; 387: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 26. Heerspink HJ, Stefánsson BV, CorreaRotter R, Chertow GM, Greene T, Hou FF, Mann JF, McMurray JJ, Lindberg M, Rossing P, Sjöström CD. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 27. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 28. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 29. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 31. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 32. Cannon CP, Pratley R, Dagogo‐Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 33. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZ. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021; 384: 129–139. [DOI] [PubMed] [Google Scholar]
- 35. Yu J, Arnott C, Neuen BL, Heersprink HL, Mahaffey KW, Cannon CP, Khan SS, Baldridge AS, Shah SJ, Huang Y, Li C. Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS program. ESC Heart Failure. 2021; 8: 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarraju A, Li J, Cannon CP, Chang TI, Agarwal R, Bakris G, Charytan DM, de Zeeuw D, Greene T, Heerspink HJ, Levin A. Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: results from the CREDENCE trial. Am Heart J. 2021; 233: 141–148. [DOI] [PubMed] [Google Scholar]
- 37. Januzzi JL Jr, Xu J, Li J, Shaw W, Oh R, Pfeifer M, Butler J, Sattar N, Mahaffey KW, Neal B, Hansen MK. Effects of canagliflozin on amino‐terminal pro–B‐type natriuretic peptide: implications for cardiovascular risk reduction. J Am Coll Cardiol. 2020; 76: 2076–2085. [DOI] [PubMed] [Google Scholar]
- 38. Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2019; 139: 2591–2593. [DOI] [PubMed] [Google Scholar]
- 39. Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018; 138: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner‐Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018; 6: 691–704. [DOI] [PubMed] [Google Scholar]
- 41. Heerspink HJ, Cherney D, Postmus D, Stefánsson BV, Chertow GM, Dwyer JP, Greene T, Kosiborod M, Langkilde AM, McMurray JJ, Correa‐Rotter R. A pre‐specified analysis of the Dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA‐CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022; 101: 174–184. [DOI] [PubMed] [Google Scholar]
- 42. Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021; 6: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McMurray JJ, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa‐Rotter R, Chertow GM, Hou FF, Rossing P, Sjöström CD, Solomon SD. Effects of dapagliflozin in patients with kidney disease, with and without heart failure. Heart Failure. 2021; 9: 807–820. [DOI] [PubMed] [Google Scholar]
- 44. Zelniker TA, Raz I, Mosenzon O, Dwyer JP, Heerspink HH, Cahn A, Goodrich EL, Im K, Bhatt DL, Leiter LA, McGuire DK. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2021; 6: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Docherty KF, Jhund PS, Claggett B, Ferreira JP, Bengtsson O, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P. Extrapolating long‐term event‐free and overall survival with dapagliflozin in patients with heart failure and reduced ejection fraction: an exploratory analysis of a phase 3 randomized clinical trial. JAMA Cardiol. 2021; 6: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butt JH, Docherty KF, Petrie MC, Schou M, Kosiborod MN, O’Meara E, Katova T, Ljungman CE, Diez M, Ogunniyi MO, Langkilde AM. Efficacy and safety of dapagliflozin in men and women with heart failure with reduced ejection fraction: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in heart failure trial. JAMA Cardiol. 2021; 6: 678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Solomon SD, Jhund PS, Claggett BL, Dewan P, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Inzucchi SE, Desai AS. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA‐HF trial. Heart Failure. 2020; 8: 811–818. [DOI] [PubMed] [Google Scholar]
- 48. Docherty KF, Jhund PS, Anand I, Bengtsson O, Böhm M, de Boer RA, DeMets DL, Desai AS, Drozdz J, Howlett J, Inzucchi SE. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA‐HF. Circulation. 2020; 142: 1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zelniker TA, Morrow DA, Mosenzon O, Goodrich EL, Jarolim P, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding J, Bode C. Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without sodium–glucose co‐transporter 2 inhibitor therapy in DECLARE‐TIMI 58. Eur J Heart Fail. 2021; 23: 1026–1036. [DOI] [PubMed] [Google Scholar]
- 50. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RH, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 51. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJ, Zelniker TA, Dwyer JP, Bhatt DL. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019; 7: 606–617. [DOI] [PubMed] [Google Scholar]
- 52. Cahn A, Mosenzon O, Wiviott SD, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JP. Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE–TIMI 58 study. Diabetes Care. 2020; 43: 468–475. [DOI] [PubMed] [Google Scholar]
- 53. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR‐reduced trial. Circulation. 2021; 143: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Januzzi JL Jr, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, Ferreira JP, Sattar N, Verma S, Vedin O, Schnee J. Prognostic importance of NT‐proBNP and effect of empagliflozin in the EMPEROR‐reduced trial. J Am Coll Cardiol. 2021; 78: 1321–1332. [DOI] [PubMed] [Google Scholar]
- 55. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐preserved trial. Circulation. 2021; 144: 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ferreira JP, Zannad F, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Jamal W, Steubl D, Schueler E, Packer M. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR‐reduced. J Am Coll Cardiol. 2021; 77: 1397–1407. [DOI] [PubMed] [Google Scholar]
- 57. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, Iwata T. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR‐reduced trial. J Am Coll Cardiol. 2021; 77: 1381–1392. [DOI] [PubMed] [Google Scholar]
- 58. Verma S, Sharma A, Zinman B, Ofstad AP, Fitchett D, Brueckmann M, Wanner C, Zwiener I, George JT, Inzucchi SE, Butler J. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across thrombolysis in myocardial infarction risk score for heart failure in diabetes categories: post hoc analysis of the EMPA‐REG OUTCOME trial. Diabetes Obes Metab. 2020; 22: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fitchett D, Inzucchi SE, Wanner C, Mattheus M, George JT, Vedin O, Zinman B, Johansen OE. Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA‐REG OUTCOME® trial. Eur Heart J. 2020; 41: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inzucchi SE, Fitchett D, Jurišić‐Eržen D, Woo V, Hantel S, Janista C, Kaspers S, George JT, Zinman B, EMPA‐REG OUTCOME® Investigators . Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose‐lowering therapy? Diabetes Obes Metab. 2020; 22: 631–639. [DOI] [PubMed] [Google Scholar]
- 61. Wanner C, Heerspink HJ, Zinman B, Inzucchi SE, Koitka‐Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, von Eynatten M, Groop PH. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA‐REG OUTCOME trial. J Am Soc Nephrol. 2018; 29: 2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018; 137: 119–129. [DOI] [PubMed] [Google Scholar]
- 63. Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA‐REG OUTCOME® trial. Eur Heart J. 2018; 39: 363–370. [DOI] [PubMed] [Google Scholar]
- 64. Dagogo‐Jack S, Cannon CP, Cherney DZ, Cosentino F, Liu J, Pong A, Gantz I, Frederich R, Mancuso JP, Pratley RE. Cardiorenal outcomes with ertugliflozin assessed according to baseline glucose‐lowering agent: an analysis from VERTIS CV. Diabetes Obes Metab. 2022; 24: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cherney DZ, Charbonnel B, Cosentino F, Dagogo‐Jack S, McGuire DK, Pratley R, Shih WJ, Frederich R, Maldonado M, Pong A, Cannon CP. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021; 64: 1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cosentino F, Cannon CP, Cherney DZ, Masiukiewicz U, Pratley R, Dagogo‐Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, Terra SG. Efficacy of ertugliflozin on heart failure–related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020; 142: 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szarek M, Bhatt DL, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH. Effect of sotagliflozin on total hospitalizations in patients with type 2 diabetes and worsening heart failure: a randomized trial. Ann Intern Med. 2021; 174: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 68. Butt JH, Docherty KF, Jhund PS, De Boer RA, Böhm M, Desai AS, Howlett JG, Inzucchi SE, Kosiborod MN, Martinez FA, Nicolau JC. Dapagliflozin and atrial fibrillation in heart failure with reduced ejection fraction: insights from DAPA‐HF. Eur J Heart Fail. 2022; 24: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amerena J. Outcomes of Empagliflozin in patients with heart failure with a preserved ejection fraction and atrial fibrillation or atrial flutter: insights from EMPEROR‐preserved. Heart Lung Circ. 2022; 31: S93–S94. [Google Scholar]
- 70. Zeng S, Delic D, Chu C, Xiong Y, Luo T, Chen X, Gaballa MMS, Xue Y, Chen X, Cao Y, Hasan AA, Stadermann K, Frankenreiter S, Yin L, Krämer BK, Klein T, Hocher B. Antifibrotic effects of low dose SGLT2 inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed Pharmacother. 2022; 146. [DOI] [PubMed] [Google Scholar]
- 71. Zeng S, Delic D, Chu C, Xiong Y, Luo T, Chen X, Gaballa MM, Xue Y, Chen X, Cao Y, Hasan AA. Antifibrotic effects of low dose SGLT2 inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed Pharmacother. 2022; 146. [DOI] [PubMed] [Google Scholar]
- 72. Chu C, Delić D, Alber J, Feger M, Xiong Y, Luo T, Hasan AA, Zeng S, Gaballa MMS, Chen X, Yin L, Klein T, Elitok S, Krämer BK, Föller M, Hocher B. Head‐to‐head comparison of two SGLT‐2 inhibitors on AKI outcomes in a rat ischemia‐reperfusion model. Biomed Pharmacother. 2022; 153. [DOI] [PubMed] [Google Scholar]
- 73. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, Borleffs CJ. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022; 28: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed search strategy on PubMed/Medline database.
Appendix S2. Detailed search strategy on Cochrane Central Register of Controlled Trials (CENTRAL) database.
Appendix S3. Search strategy from ClinicalTrials.gov.
Appendix S4. Numbers of included randomized controlled trials registered at ClinicalTrials.gov registry.
Appendix S5. Effects of SGLT2 inhibitors therapy on atrial fibrillation in heart failure patients: results from meta‐analysis.
Appendix S6. Common side effects of the SGLT2 inhibitors: results from meta‐analysis.
Appendix S7. Effects of the SGLT2 inhibitors on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S8. Effects of the GLP‐1 agonists on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S9. Effects of the DPP‐4 inhibitors on HHF, CV death, and MACE: results from meta‐analysis.
Appendix S10. Publication bias analysis: results from meta‐analysis.
Appendix S11. Effects of SGLT2 inhibitors on end‐stage renal disease (ESRD) and publication bias analysis: results from meta‐analysis.


