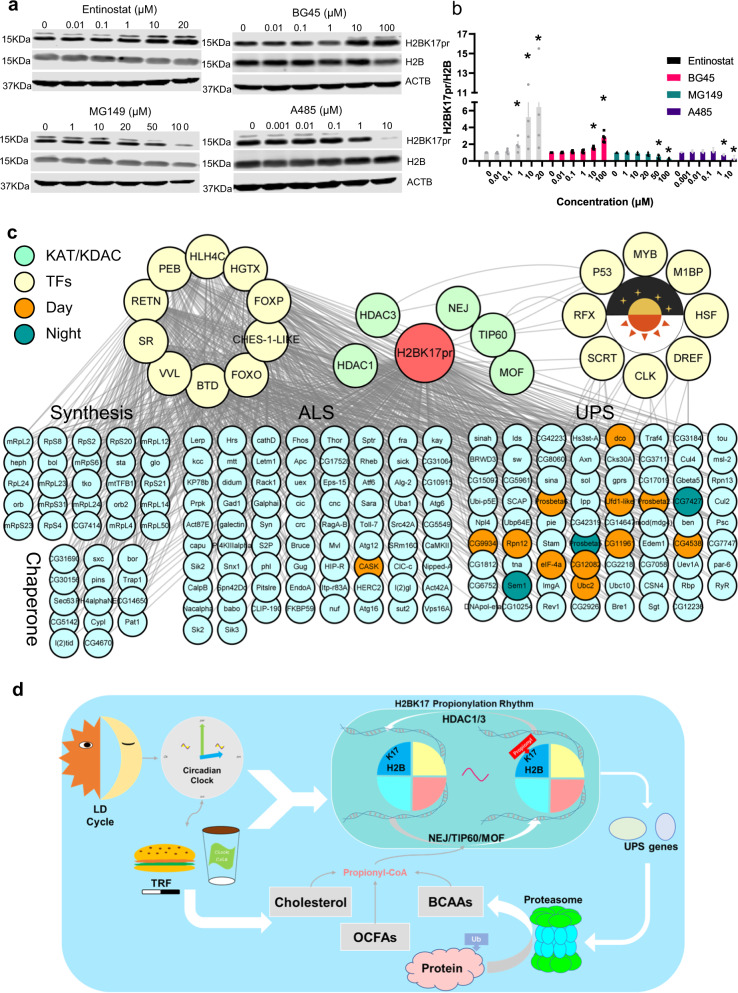
Fig. 7. The potential regulatory mechanism of H2BK17pr.
a Representative Western blots of protein extracts from S2 cells treated with Entinostat, BG45, MG149 and A485 under indicated concentrations. ACTB is used as a loading control. The blotting experiments were conducted with three independent repeats. b Histogram shows quantification of normalized H2BK17pr level in (a). The normalized intensity at 0 μM is set to 1 (two-tailed Mann–Whitney U test for unpaired comparisons. Entinostat, n = 4 biologically independent experiments, * p = 0.02107; BG45, n = 5 biologically independent experiments, * p = 0.00749; MG149, n = 4 biologically independent experiments, * p = 0.02107; A485, n = 4 biologically independent experiments, * p = 0.02107). c A H2BK17pr-centered signaling network including the 5 potential regulators (in light green) and PN genes differentially expressed in H2BK17A flies, as well as 18 TFs (in yellow) predicted to be involved. The 10 TFs on the left were predicted to regulate genes with constant H2BK17pr binding while the 8 TFs on the right were predicted to regulate genes with rhythmic H2BK17pr binding. Orange circle indicates genes with peak H2BK17pr binding during the day, while dark green circle indicates genes with peak H2BK17pr binding at night. d Model illustrating the regulatory mechanism of H2BK17pr, which in turn modulates the expression of UPS genes that ultimately influence protein degradation. Data are presented as the mean ± SD. Source data are provided as a Source Data file.