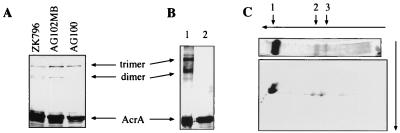
FIG. 2.
Inner-membrane-associated AcrA is a trimer. (A) DSG cross-linking experiments demonstrating that oligomerization of AcrA is independent of AcrB and TolC. AcrA was stained with anti-AcrA antibody. (B) Cross-linked oligomeric AcrA after purification on an Ni2+ affinity column. AcrA was expressed as OmpA-AcrA-His6 fusion protein from pOA. After cross-linking with DSP, AcrA-His6 was purified and analyzed by Western blotting with the anti-AcrA antibody. Treatment with dithiothreitol (lane 2), which reverses DSP cross-links, confirmed that the purified AcrA forms are products of cross-linking. (C) Analysis of the cross-linked AcrA by two-dimensional SDS-PAGE demonstrating that AcrA (arrow 1) is the only component of complexes CA1 (arrow 3) and CA2 (arrow 2). The proteins were stained with Coomassie blue.