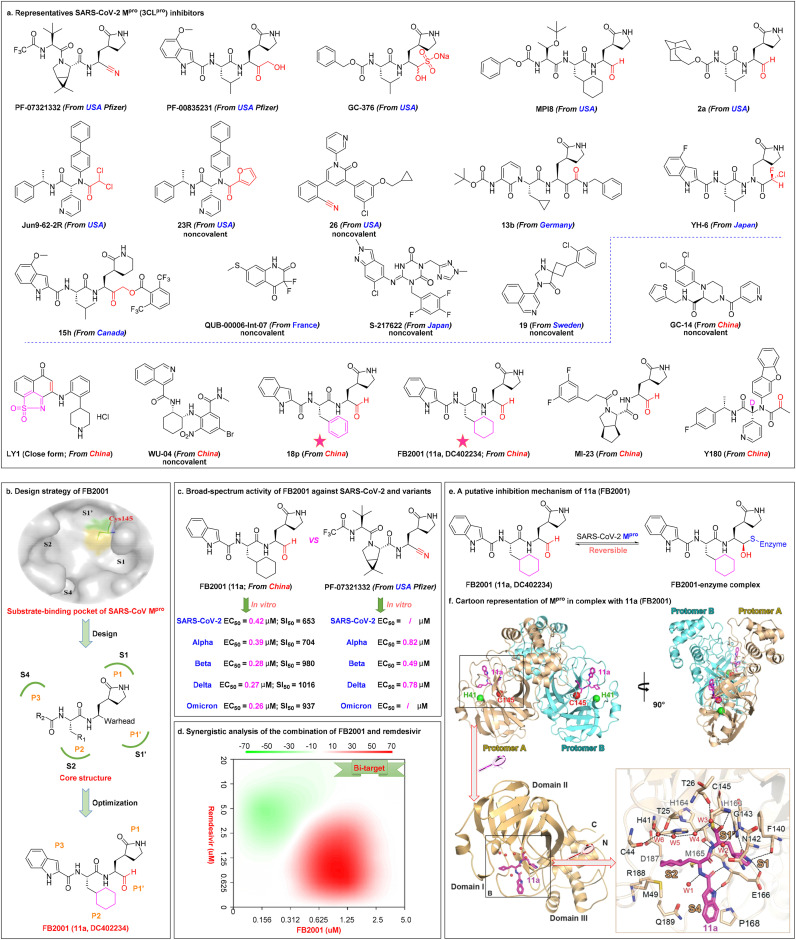
Fig. 5.
Representative SARS-CoV-2 Mpro(3CLpro) inhibitors and China's first inhaled aerosolized SARS-CoV-2 Mproclinical candidate FB2001. (a) Chemical structures of selected SARS-CoV-2 Mpro inhibitors (PF-07321332, PF-00835231, GC-376, MPI8, 2a, Jun9–62-2R, 23R, and 26 from USA; 15h from Canada; 13b from Germany; QUB-00006-Int-07 from France; YH-6 and S-217622 from Japan; 19 from Sweden; GC-14, LY1, WU-04, 18p, FB2001, MI-23, and Y180 from China) for COVID-19 treatment. (b) The design strategy of FB2001 through analysis of the substrate-binding pocket of SARS-CoV Mpro to guide design of SARS-CoV-2 Mpro inhibitors (based on the high degree of structural homology and similar substrate specificity of Mpro between SARS-CoV-2 and SARS-CoV). (c) Broad-spectrum activity of FB2001 against SARS-CoV-2 and variants, and comparison of activity between FB2001 with US FDA-approved PF-07321332. (d) Synergistic analysis of the combination of FB2001 and remdesivir. In cases where the synergy score <−10 the interaction was likely to be antagonistic, −10 < synergy score <10 indicated an additive effect, and synergy score >10 indicate a synergistic effect. (e) Schematic representation of the inhibitory mechanism of FB2001. The aldehyde carbon of FB2001 reacts reversibly with the nucleophilic sulfur atom of Cys145, thus forming a covalently bound tetrahedral complex. (f) The structure of SARS-CoV-2 Mpro in complex with FB2001. Cartoon representation of Mpro in complex with FB2001 in two different views. The catalytic dyad (His41 and Cys145) is indicated as green and red spheres, respectively. FB2001 is shown as magenta sticks. Reproduced with permission. Copyright 2022, Elsevier B.V.