Fig. 3.
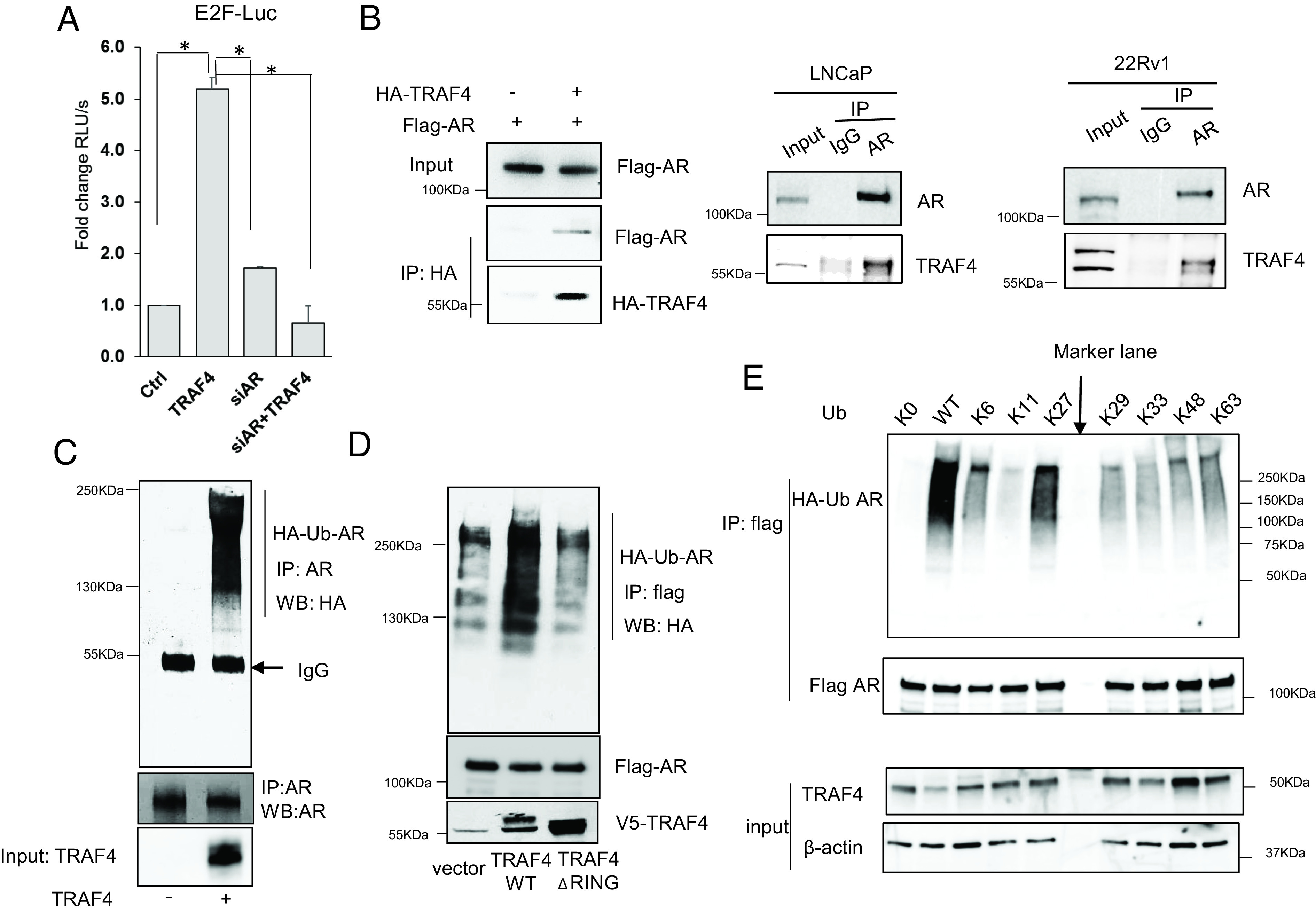
TRAF4 mediates atypical K27-linked AR polyubiquitination. (A) AR is important in mediating the activation effect of TRAF4 on E2F activity. LNCaP cells were treated with control or siAR, and then transiently transfected with an E2F-driven luciferase reporter and a TRAF4 expression plasmid. Shown is the luciferase reporter activity. (B) TRAF4 interacts with AR in cells. Left, Flag-AR was transiently transfected into 293T cells in the absence or presence of HA-TRAF4 cotransfection. A coimmunoprecipitation (co-IP) experiment was then carried out using an HA-specific antibody. Middle and Right, endogenous AR interacts with endogenous TRAF4 in LNCaP and 22Rv1 cells, respectively. AR was immunoprecipitated using an AR-specific Ab or IgG control followed by western blot analysis. (C) TRAF4 promotes AR in vitro ubiquitination. Purified recombinant AR protein was incubated with purified UBE1, UbcH5a, HA-ubiquitin in the absence or presence of purified TRAF4 protein. An IP was then carried out using an AR-specific antibody followed by western blot analysis using an HA antibody to detect AR ubiquitination. (D) TRAF4 WT but not its RING domain deletion mutant promotes AR ubiquitination in cells. Flag-AR and HA-ubiquitin, V5-WT TRAF4, ΔRING mutant or vector control were transiently transfected into 293T cells followed by IP using a flag antibody. The levels of AR ubiquitination were measured using an HA antibody in a western blot analysis. (E) TRAF4-mediated AR ubiquitination mainly occurs through the K27-linkage. K0 represents all lysine residues in ubiquitin were mutated. K6 to K63 represents the ubiquitin mutant with all lysine mutations except the depicted number of lysine. Flag AR, V5-TRAF4, and different HA-ubiquitin (WT or mutants) were transiently transfected into 293T cells. An IP experiment was carried out using a flag antibody followed by a western blot analysis to detect AR ubiquitination with an HA antibody. * represents P < 0.05.
