Abstract
Background: Aortic valve stenosis is an important clinical condition, with a significant mortality rate in the elderly. Plasma values of alkaline phosphatase (ALP) have been shown to act as a marker of prognosis in different clinical conditions and in the general population.
Methods: Plasma levels of ALP were studied in a cohort of patients with aortic valve stenosis, and a 5-year survival evaluation was performed.
Results: Twenty-four patients were under study, of whom 12 were dead at the 5-year follow-up. The median age at baseline evaluation was 79 years (interquartile range, 72–85 years), and 11 patients were female (13 were male). The median value of ALP, of 83 IU/L, was used to separate patients into two groups: 2 patients who died in the group with low ALP values versus 10 patients who died in the group with high ALP values. Using ALP with the same cutoff level, the Kaplan–Meier study with log-rank analysis showed a significance level <0.01. Cox regression analysis showed an overall significant result, with a significant level for plasma ALP (significance level 0.03), but not for age, sex, or transvalvular gradient (assessed by echocardiography).
Conclusions: Elevated plasma ALP is associated with increased mortality risk in patients with aortic valve stenosis. This finding may merit evaluation in studies with a larger number of patients.
Keywords: alkaline phosphatase, aortic valve stenosis, mortality
Introduction
Aortic valve stenosis is a clinical condition which is growing in importance because many human populations across the globe show an increase in their average age, and this is a disease that appears mainly in the elderly. The diagnosis of this disease rests mainly, although not exclusively, on echocardiography. Aortic valve stenosis in the elderly is a disease with a significant mortality rate.1 Aortic valve stenosis is currently categorized as severe (in the case of typical high gradient aortic stenosis) if echocardiography shows mean transvalvular gradient ≥40 mmHg, peak velocity ≥ 4.0 m/s, and valve area ≤1 cm2 (or ≤0.6 cm2/m2).2
Alkaline phosphatase (ALP) is a hydrolase that removes phosphate groups from different types of molecules, acting as an ectoenzyme (being attached to the outer cell membrane). Different isoenzymes are currently recognized: tissue nonspecific, intestinal, placental, and germ cell.3 ALP is important for bone mineralization; deficient activity of ALP of genetic origin leads to the clinical condition of hypophosphatasia.4 In a study involving 4155 adults, the authors found an association between ALP and age, waist circumference, body mass index, blood pressure, exercise, alcohol intake, triglycerides, other liver enzymes, cardiovascular disease, arterial hypertension, hypercholesterolemia, and diabetes mellitus.5 Zhong et al6 found that ALP levels were associated with increased mortality in patients with acute ischemic stroke. In patients with coronary artery disease, ALP was associated with increased mortality, both in patients with myocardial infarction and diabetes mellitus7 and in patients who underwent coronary angioplasty with stent implantation.8 Moreover, ALP is currently seen as an independent predictor of mortality in the general population.9
In the present report, the aim was to study the relation between plasma ALP and survival in a small cohort of patients with aortic valve stenosis.
Methods
Consecutive patients with a diagnosis of aortic valve stenosis were enrolled to this study, and this study was performed in a general cardiology outpatient clinic. Baseline evaluation included echocardiography, electrocardiography, and blood tests, including ALP.
In the present report, 5-year (60 months) survival was under study, and this was established prospectively by the study of electronic health records, after a minimum period of 55 months for each patient had passed from the initial evaluation. In the case of patients who died, the date of death was recorded, when available, or alternatively, the date of the last observation of each patient was used. In the case of patients not known to be dead, censoring was performed in the date of the last observation. No attempt was made to study the causes of death.
The two groups of patients (dead or alive) were compared by means of (the nonparametric) Mann–Whitney U test, considering age, mean left ventricular/aortic gradient (as measured by echocardiography; mean transvalvular gradient), and plasma ALP.
The median value for ALP in the present cohort under study (83 International Units/Liter [IU/L]) was used as cutoff, and the Kaplan–Meier study was performed. The comparison between groups was made using the log-rank test.
Cox proportional hazards survival modeling was used. Covariates included sex, age, plasma ALP, and mean transvalvular gradient. The relative risk for mortality was calculated by dividing the patients in two groups according to the median ALP value.
A significance level of 0.05 or lower was considered statistically significant. Data analysis was performed using SPSS 26 software program, from IBM (Amonk, NY), except for relative risk calculation, for which the MedCalc online calculator was used (available at https://www.medcalc.org/calc/relative_risk.php).
The present research project was approved by the ethics committee of our institution. Informed consent to participate in this study was obtained from participants.
Statement of ethics
This research complies with internationally accepted standards for research practice and reporting. The present research project was performed under an authorization granted from the Institutional Ethics Committee.
Results
Two patients were not enrolled in this study because of lack of willingness to sign the informed consent form. A total number of 24 patients were under study, of an initial number of 27 patients. Three patients were excluded because of not having performed blood studies at our institution. Baseline data are presented in Table 1.
Table 1.
Baseline characteristics of the patients with aortic valve stenosis cohort under study
| Variable | Median | Interquartile range |
|---|---|---|
| Age (years) | 79 | 72–85 |
| Transvalvular gradient (mm Hg) | 35 | 31–46 |
| Plasma ALP (IU/L) | 83 | 57–115 |
| Sex | 11 Female patients | 13 Male patients |
ALP, alkaline phosphatase; IU/L, International Units/Liter.
A total number of 7 patients underwent aortic valve intervention during the 5-year follow-up (surgery in 6 patients and percutaneous valve implantation in the remaining case). These 7 patients had, cumulatively, a severe degree of valve stenosis (as evaluated by echocardiography), willingness to allow valve intervention, and acceptance from the part of the surgical/cardiac hemodynamic team. These seven patients were alive at the end of follow-up.
Concerning electrocardiography, two patients had atrial fibrillation, one had atrial flutter, and the remaining patients were in sinus rhythm.
At the 5-year follow-up, 12 patients were dead and 12 were alive (one patient in this latter group had a 56-month follow-up, unlike all others, with a 60-month follow-up). Two patients died in the group with low ALP values versus 10 patients who died in the group with high ALP values.
The Mann–Whitney U test showed that age (P < .01) and plasma ALP (P < .01) were significantly higher in dead patients, when compared with patients living at the 5-year follow-up; the same did not happen in the case of mean transvalvular gradient (P = .13).
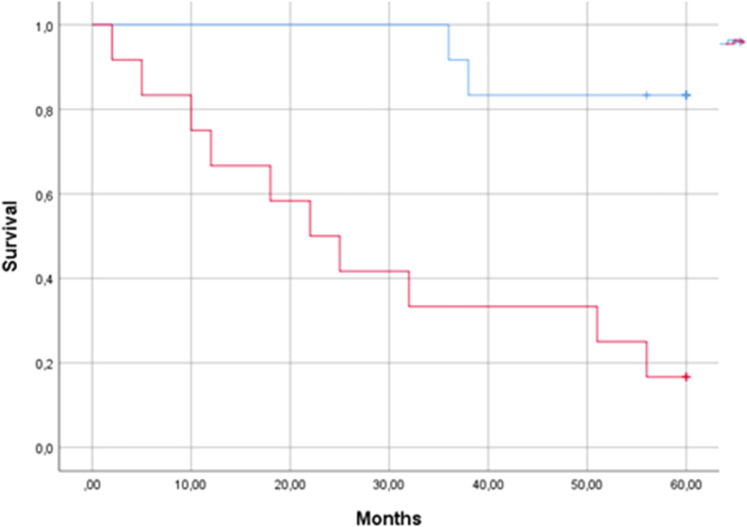
As shown in Fig. 1, patients with an ALP plasma level above the median value had a marked mortality rate, unlike other patients. The Kaplan–Meier study with the log-rank test showed an overall significance level <0.01.
Figure 1.
Kaplan–Meier survival curves for patients with aortic valve stenosis with plasma alkaline phosphatase below (top line, 12 patients) or above (bottom line, 12 patients) the median value of 83 IU/L.
A relative risk of 5.0 was seen for mortality for patients with increased ALP levels when compared with patients with lower levels (95% confidence interval of 1.4–18.2).
Cox regression analysis showed an overall significant result, with a significant level for plasma ALP (Table 2). Similar findings were seen after inserting an additional variable (valve intervention) in the model (the new variable was not significant in this model; data not shown).
Table 2.
Significance levels for Cox regression analysis of the patients with aortic valve stenosis cohort under study
| Variable | Significance level |
|---|---|
| Overall | <0.01 |
| Sex | 0.47 |
| Age (years) | 0.17 |
| Transvalvular gradient (mm Hg) | 0.26 |
| Plasma ALP (IU/L) | 0.03 |
ALP, alkaline phosphatase; IU/L, International Units/Liter.
Discussion
In the present report, elevated plasma ALP was shown to be able to identify patients with aortic valve stenosis with a high mortality rate. Owing to the wide availability of this biomarker, ALP could be established as a useful aid in the management of this type of patient. As stated above, elevated ALP has been shown to act as a negative prognostic factor both in patients with heart disease and in the general population,7,8,10 and ALP is currently seen as an independent predictor of mortality in the general population.9
The mechanism underlying the increased mortality seen in patients with elevated ALP levels is unclear at the present stage. Vascular calcification is frequently presented as a consequence of increased ALP activity because the enzyme hydrolyzes pyrophosphate, an inhibitor of vascular calcification.10 In the case of patients with aortic valve stenosis, increased valve calcification could exist in the setting of increased ALP levels. Clark-Greuel et al11 showed that ALP gene expression was increased in some, but not all cases of human calcified valves. These authors also showed that ALP was increased in experimental (cell culture) stimulation of calcification of aortic valve interstitial cells.
ALP generates inorganic phosphate,9 is present in myocardial capillary endothelial cells,12 and may modulate inflammation,9 which may also play a part in the context under study. The possibility that changes in the liver associated with hemodynamic changes lead to increased ALP levels cannot be ruled out at the present stage. ALP levels could vary in the setting of both the liver and systemic diseases.
Several types of biomarkers have been studied in patients with aortic valve stenosis, including cardiac troponins and natriuretic peptides, and have been shown to correlate with patient mortality.13 A major interest of biomarker studies in aortic valve stenosis, apart from evaluating prognosis, would be to guide the ideal time frame for valve intervention, a topic possibly to be explored in clinical trials, in the case of ALP.
Furthermore, one may speculate that this study of intrinsic mechanisms by which increased ALP is associated with increased mortality could allow the finding of useful interventions to decrease mortality in these patients and in others.
Study limitations
This study has significant limitations, the main of which is that the small dimension of the sample limits the strength of conclusions. This small cohort includes only patients with a relatively advanced age (range of 65–93 years at baseline evaluation), and therefore, the findings may not be applicable to younger patients.
Conclusions
Elevated plasma ALP is associated with increased mortality risk in patients with aortic valve stenosis. This finding may merit evaluation in studies with a larger number of patients.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgments
A preprint version of this text is available at MedRxiv (doi: https://doi.org/10.1101/2022.03.15.22272399).
References
- [1].Varadarajan P, Kapoor N, Bansal RC, Pai RG. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged ≥80 years. Eur J Cardiothorac Surg. 2006;30:722–7. [DOI] [PubMed] [Google Scholar]
- [2].Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- [3].Van Hoof VO, De Broe ME. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci. 1994;31:197–293. [DOI] [PubMed] [Google Scholar]
- [4].Moore CA, Ward JC, Rivas ML, Magill HL, Whyte MP. Infantile hypophosphatasia: autosomal recessive transmission to two related sibships. Am J Med Genet. 1990;36:15–22. [DOI] [PubMed] [Google Scholar]
- [5].Webber M, Krishnan A, Thomas NG, Cheung BM. Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and Nutrition Examination Survey 2005-2006. Clin Chem Lab Med. 2010;48:167–73. [DOI] [PubMed] [Google Scholar]
- [6].Zhong C, You S, Chen J, et al. Serum alkaline phosphatase, phosphate, and in-hospital mortality in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2018;27:257–66. [DOI] [PubMed] [Google Scholar]
- [7].Nunes JPL, Melao F, Godinho AR, Rodrigues JD, Maciel MJ. Plasma alkaline phosphatase and survival in diabetic patients with acute myocardial infarction. Ann Transl Med. 2016;4:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park JB, Kang DY, Yang HM, et al. Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug-eluting stent. Eur Heart J. 2013;34:920–31. [DOI] [PubMed] [Google Scholar]
- [9].Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P. Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol. 2017;13:429–42. [DOI] [PubMed] [Google Scholar]
- [10].Tonelli M, Curhan G, Pfeffer M, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–92. [DOI] [PubMed] [Google Scholar]
- [11].Clark-Greuel JN, Connolly JM, Sorichillo E, et al. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83:946–53. [DOI] [PubMed] [Google Scholar]
- [12].Schultz-Hector S, Balz K, Bohm M, Ikehara Y, Rieke L. Cellular localization of endothelial alkaline phosphatase reaction product and enzyme protein in the myocardium. J Histochem Cytochem. 1993;41:1813–21. [DOI] [PubMed] [Google Scholar]
- [13].White M, Baral R, Ryding A, et al. Biomarkers associated with mortality in aortic stenosis: a systematic review and meta-analysis. Med Sci. 2021;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]