Abstract
Objective:
To analyse the cost-effectiveness of Baby-Friendly Hospital Initiative (BFHI) in promoting breast-feeding during the first hour of life (BFFHL) and reducing late neonatal mortality.
Design:
Cost-effectiveness economic assessment from the health system perspective, preceded by a prospective cohort of mother–baby followed from birth to 6 months of life. The direct costs associated with two health outcomes were analysed: intermediate end point (BFFHL) and final end point (reduction in late neonatal mortality).
Setting:
Study was carried out in six hospitals in the city of São Paulo (Brazil), three being Baby-Friendly Hospitals (BFH) and three non-BFH.
Participants:
Mothers with 24 h postpartum, over 18 years old, single fetus and breast-feeding at the time of the interview were included. Poisson regressions adjusted for maternal age and level of education were estimated to identify factors related to BFFHL and late neonatal mortality. Sensitivity analysis was performed to ensure robustness of the economic assessment.
Results:
Cost-effectiveness analysis showed that BFHI was highly cost-effective in raising BFFHL by 32·0 % at lower cost in comparison with non-BFHI. In addition, BFHI was cost-effective in reducing late neonatal mortality rate by 13·0 % from all causes and by 13·1 % of infant mortality rate from infections.
Conclusions:
The cost-effectiveness of the BFHI in promoting breast-feeding and reducing neonatal mortality rates justifies the investments required for its expansion within the Brazilian health system.
Keywords: Breast-feeding, Neonatal mortality, Baby-friendly, Cost-benefit analysis, Brazil
Breast-feeding ensures food and nutrition in early life years with long-term benefits on health throughout individuals’ lifetime. In addition, its benefits are extensive amidst infants and mothers from high-, medium- and low-income population groups. It is considered a health investment with lasting effects in physical, cognitive and social development of infants, usually resulting in improvements of intergenerational formation of human capital(1,2).
Policies designed to protect, promote and support breast-feeding are considered strategies that favour enduring health, social and economic benefits within national health systems(1–3); thus, it is important to assess its short-term costs and effectiveness to ensure the sustainability of programmes independently of political contexts. The Baby Friendly Hospital Initiative (BFHI) was established by the WHO and the UNICEF to incentivise breast-feeding by reorienting practices and childbirth routines within health facilities, following the Ten Steps to Successful Breastfeeding(4,5).
There is significant evidence on the role of BFHI for breast-feeding promotion(4–9); however, unfortunately, estimates point that only 10·0 % of births occur in BFHI facilities worldwide(4). The prevalent low BFHI coverage results from diverse barriers lead to scale up the initiative within different countries, especially challenges related to large-scale implementation due to lack of cost estimates at the national level(10), criticism on the effects of the ‘Ten Steps’ and lack of policy guidance for implementation. To tackle the challenges posed in BFHI, a recent review of the ‘Ten Steps’ was published by the WHO including supplemental material to comprise implementation guidelines to support governments towards scaling up the strategy(4,5,9).
In Brazil, the BFHI was adopted as a public policy within the health system since 1992; yet the current coverage is only 23·4 % of births in BFHI facilities nationwide(7,11), indicating the relevance of addressing the programme implementation challenges. A recent analysis conducted in Brazil indicated that BFHI is effective in reducing infant mortality by promoting breast-feeding(12); nevertheless, there is still a gap in the literature regarding the costs associated with adoption of BFHI (i.e., whether effects outweigh financial resources applied in its implementation and maintenance).
The BFHI accreditation in Brazil follows the adherence of the Ten Steps to Successful Breastfeeding and additional criteria. These include the compliance to the Brazilian Standard for the Trading of Marketing of Food for Infants and Early Childhood, Pacifiers, Baby Bottles and Nipple Shields, the global Woman-Friendly Care criterion and the assurance of free access and/or stay of an accompanying person with the newborn 24 h/d(11). In 2014, the Ministry of Health reformulated the criteria to enable hospitals to get the BFHI accreditation, excluding the mandatory criterion on low rate of caesarean sections and including financial incentives based on higher reimbursements for adherence of public hospitals to best practices during birth, thus encouraging vaginal delivery. Hospitals accredited as BFHI receive a monthly financial transfer from the federal government.
Both WHO(13) and the Brazilian Ministry of Health(14) recommend that economic assessments should be performed in order to incorporate innovative practices and protocols in health services. Scientific evidence on the cost and effectiveness of health interventions should support decision-making processes in order to promote the optimisation of resources for health systems(2). Therefore, the aim of the study was to perform an economic assessment of BFHI in promoting breast-feeding and reducing neonatal mortality in Brazil, using cost-effectiveness analysis to indicate the costs and the effects that justify investments for maintenance and scale-up of the BFHI.
Methodology
Study design
Study conducted with data from a prospective cohort including mother−infant pairs selected in six hospitals in São Paulo (Brazil) from 2016 to 2018 and monitored from birth to 6 months of life. Then, the researchers performed a cost-effectiveness analysis based on the direct costs estimation of hospitals that adopt the BFHI (Baby-Friendly Hospitals, BFH), and its effectiveness in promoting breast-feeding and reducing late neonatal mortality, compared with hospitals that do not adopt the BFHI (non-BFH), according to the Brazilian government perspective (i.e., costs and health outcomes that are relevant to the public health system).
Sample
The hospitals included in the intervention group (BFH) were selected from the BFH that met all of the BFHI criteria in the previous year, according to the online self-monitoring of the Brazilian Ministry of Health. The three hospitals with the highest number of specialised beds (gynaecology, obstetrics, neonatal ICU and neonatology) were included, thus representing 25 % of the hospitals participating in the initiative in São Paulo. Then, three non-BFH were selected to compose the paired control group. The hospitals included in the control group were selected according to the main similar characteristics in relation to the hospitals in the intervention group (geographic location, state or municipal management and number of beds), to allow the comparison between two health strategies (BFH and non-BFH) for economic evaluation.
Sample size of mother−infant pairs was estimated with 95 % CI, 80 % test power and +28 % effect on exclusive breast-feeding among BFHI-born children following estimates from previous studies(15,16), resulting in minimum sample size of 686 mother−infant pairs.
The procedure for the selection of mother−infant pairs at each hospital was based on stratified proportional probability, taking into account the number of deliveries and the proportion of caesarean sections in each hospital, according to the estimated sample size. The sample was expanded by 41 % to compensate for possible losses from follow-up, resulting in 969 mothers who were initially included in the cohort.
The inclusion criteria were interview occurring >24 h postpartum, mother with 18 years or older, single fetus and breast-feeding at the time of the interview. The exclusion criterion was delivery outside the hospital. Mothers who met the inclusion criteria were invited to participate in the study at each hospital; all mothers who agreed, read and signed the informed consent form were included in the study until the sample size was obtained.
Data collection
Data collection included four data sources: (i) structured interview with mothers, (ii) institutional protocols, (iii) medical records and (iv) interview with healthcare professionals.
First, structured interviews were conducted with mothers enrolled at the hospitals to investigate:
-
1.
Birth and birth routines related to BFHI (i.e., type of delivery, breast-feeding support in the delivery room, skin-to-skin contact and breast-feeding in the first hour);
-
2.
Child characteristics (i.e., birth weight, gestational age, gender);
-
3.
Maternal characteristics (i.e., age, per capita income, education, intention to breastfeed, colour and marital status).
Then, institutional protocols were obtained at the hospitals in order to define standard procedures and protocols during pre-delivery, delivery and postpartum until discharge, according to the health professional category.
Next, an in-depth analysis was conducted in the medical records of a subsample consisting of 14 % of the mothers in order to identify in detail the resources and supplies used in each procedure. Considering that health procedures follow protocols using combinations of inputs and generally use similar proportions of certain inputs in similar circumstances(17), the researchers initially established a subsample of 10 % of the medical records to gather information to estimate the typical direct costs of birth within each hospital. However, given the increase in the sample of mothers interviewed (+41 %), the subsample of medical records was proportionately increased to 14 %. Due to requirements of comprehensive information from pre-delivery to post-delivery discharge to perform micro-costing, the study analysed the medical records to extract the amounts and the types of examinations, supplies, medications, human resources and length of stay of the mother−infant pairs.
Lastly, health professionals involved in the protocols (e.g., doctor, nurse, nursing technician, anaesthetist and neonatologist) were interviewed as for the frequency and average time spent for each procedure to establish the adherence to protocols. The BFHI supervisors were also interviewed in order to obtain the costs of inputs used and the time spent by health professionals (according to their categories) on breast-feeding clinical management education during the previous year. The information was collected by trained researchers, then coded and typed into single data set.
Data analysis
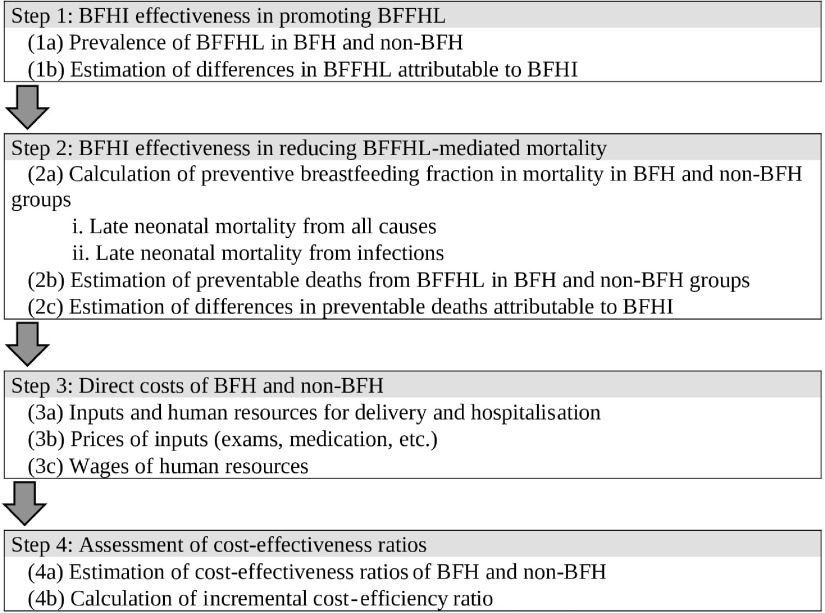
The analysis was conducted in four steps (Fig. 1), according to details described in the following sections, including data sources used and statistical tests performed. Statistical analysis was conducted using Stata software version 13.1.
Fig. 1.
Description of the analytical steps to estimate the reduction in breast-feeding-mediated late neonatal mortality in the first hour of life promoted by the Baby-Friendly Hospital Initiative (BFHI), São Paulo city, 2018. BFFHL, breast-feeding in the first hour of life; BFH, Baby-Friendly Hospital; non-BFH, non-Baby-Friendly Hospital
Baby-Friendly Hospital Initiative effectiveness in promoting breast-feeding during the first hour of life
The effectiveness of BFHI to promote breast-feeding in the first hour of life (BFFHL) (intermediate outcome) was assessed using information from mothers’ interviews. Intervention and control groups were compared based on χ2 test for comparison of proportions and the Mann–Whitney test for comparison of means. In sequence, χ2 test was used to verify associations between explanatory variables and BFFHL, and variables with P < 0·20 were eligible for inclusion in the final model. Bivariate analysis using robust Poisson regression (i.e., crude prevalence ratio, PR c ) was conducted. Maternal age and education, and variables with PR c < 0·10 were included in the final model, generating adjusted prevalence ratios (PR adj ). Children born in BFH facility showed higher prevalence of BFFHL; therefore, there was positive difference in the prevalence of BFFHL between BFH and non-BFH groups, representing the increment promoted by the BFHI.
Baby-Friendly Hospital Initiative effectiveness in reducing breast-feeding in the first hour of life-mediated mortality
The number of late neonatal deaths from all causes and from infections potentially avoided due to BFFHL in both groups was estimated using preventive fraction (PF), to obtain the differences in mortality rates (final outcome). The PF is defined as the proportion of morbidity and mortality cases avoided by exposure to protection factors and corresponds to the proportion of morbidity and mortality that would occur if there was no intervention conducted in the population(18,19), described by Miettinen(20) and used in similar studies(21,22). PF was calculated according to the following equation(21):
where P x is the prevalence of protective exposure (breast-feeding) in the sample, and RR is the relative risk on protective effect of breast-feeding in children from 7 to 27 d of life with BFFHL in comparison with children without BFFHL, obtained from Debes et al.(23). The estimated number of late neonatal deaths avoided (cases averted = CA) by BFFHL in São Paulo city during 2016 was obtained using the following equation(21):
where N x is the number of cases observed in the municipality of São Paulo in 2016(24) and PF x is the preventive fraction of the mortality indicator.
Direct costs of Baby-Friendly Hospitals and non-Baby-Friendly Hospitals
The direct costs were calculated using micro-costing technique according to the type of delivery (vaginal delivery (VD) and caesarean delivery (CS)) and length of stay (2 , 3–4 and ≥5 d), resulting in six cost categories (VD2, VD3, VD5, CS2, CS3 and CS5). The following cost items were considered in the costs calculation: human resources, examinations, supplies, medications and BFHI training.
Human resource costs were estimated using the hourly wage and the time spent by each health professional on the mother−infant pairs. The annual workload and the number of participants were considered to estimate costs related to BFHI training, using the hourly wages according to health professional categories, including the trainers. The annual cost of training was divided by the number of annual deliveries in each BFH.
The estimated mean direct costs of the subsample (14 %) were considered representative of the direct costs of other mothers within each hospital sample, according to the groups of mothers with similar type of delivery and length of stay. Thus, the mean direct costs per hospital were estimated and included details of mean direct costs per type of delivery and length of stay.
Direct costs were estimated up to June 2018, using official inflation rates in Brazil, published by the Brazilian Institute for Geography and Statistics, and converted into US dollars, applying the official exchange rates available at the Brazilian Central Bank at reference period.
Assessment of cost-effectiveness ratios
The cost-effectiveness ratios (CER) of BFH and non-BFH were calculated by dividing direct costs of births within each group by its effectiveness. Incremental cost-effectiveness ratio (ICER) was also estimated referring to the differences in direct costs between the two groups divided by the differences in health outcomes, using the following equation(14):
where C BFH is the direct costs of BFH intervention group, C NBFH is the direct costs of non-BFH comparison group, E BFH is the effectiveness of BFH intervention group and E NBFH is the effectiveness of non-BFH comparison group.
Regarding the avoided deaths (final outcome), the cost-effectiveness was estimated using decision tree modelling(14) (see Appendix 1 in the online supplementary material). CER and ICER were calculated considering probabilities of tree branches modelled according to the following parameters: delivery (born in BFH or non-BFH), type of delivery (vaginal or caesarean birth), BFFHL (yes or no), average gross cost per type of delivery and the probability of death. Thus, the final assessment was based on cost per death avoided, considering costs within each group in relation to probabilities in decision tree branches.
The probabilities of late neonatal deaths in infants breastfed or not in the first hour of life were estimated using the equation proposed by Bartick & Reinhold(25).
where X is the probability of death in non-breastfed infants, X × R is the probability of death in breastfed infants, B is the current prevalence of breast-feeding, R is the risk rate in favour of breast-feeding(23) and S is the general incidence of all-cause late neonatal mortality rate in the population (2·25 deaths per 1000 live births in São Paulo municipality in 2016)(24).
Univariate sensitivity analysis was performed for the intermediate outcome (BFHHL) and final outcome (avoided deaths) in order to verify robustness of CER and ICER according to parameter variations(14). Regarding intermediate outcome scenarios, estimations were performed adopting changes (±10 %) in the following parameters: cost per delivery, length of stay, human resources, medications, inputs, tests and effectiveness (BFFHL). Referring to final outcome scenarios, sensitivity analysis was based on variations (±10 %) of parameters included in decision tree branches (group, average cost, type of delivery and effectiveness (deaths avoided)). The results of the sensitivity analysis are shown in dispersion diagrams and tornado diagrams, thus providing analyses of several parameters at the same time. This method allows visualising the magnitude of the impact on the ICER of each of the simulated scenarios with the variation in costs or final outcomes.
Results
Baby-Friendly Hospital Initiative effectiveness
The sample used for the cost-effectiveness analysis consisted of 642 mother−infant pairs who reported the length of stay: 58·3 % in the BFH group and 41·7 % in the non-BFH group (see Appendix 2 in the online supplementary material). The variables related to the mother (age, education and work), the child (gestational age, birth weight and type of delivery) and per capita income were similar between both groups. Table 1 presents the sample characteristics that showed significant differences between the BFH and non-BFH groups.
Table 1.
Characterisation of the individuals in the sample, according to groups, São Paulo city, 2018
| Characteristics | BFH | Non-BFH | P * | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Delivery routines | |||||
| Type of delivery (n) | 375 | 267 | 0·365 | ||
| Vaginal | 62·1 | 66·7 | |||
| Caesarean | 36·3 | 32·6 | |||
| Skin-to-skin contact | 375 | 267 | 0·000 | ||
| Yes | 32·3 | 3·4 | |||
| No | 67·7 | 96·6 | |||
| BFFHL | 375 | 267 | 0·000 | ||
| Yes | 66·7 | 50·6 | |||
| No | 33·3 | 49·5 | |||
| Maternal characteristics | |||||
| Intention to breastfeed | 369 | 265 | 0·000 | ||
| Yes | 53·7 | 67·5 | |||
| No | 46·3 | 32·5 | |||
| Skin colour | 362 | 259 | 0·026 | ||
| Black | 24·6 | 17·4 | |||
| Brown | 33·4 | 49·4 | |||
| White | 42·0 | 33·2 | |||
| Living with partner | 369 | 266 | 0·000 | ||
| Yes | 85·4 | 69·5 | |||
| No | 14·6 | 30·5 | |||
BFH, Baby-Friendly Hospital; non-BFH, non-Baby-Friendly Hospital; BFFHL, breast-feeding in the first hour of life.
χ2 test comparing intervention and control groups.
Regarding the delivery routines, skin-to-skin contact and BFFHL were more prevalent in the BFH group. In relation to maternal characteristics, the non-BFH group had more mothers who were intending to breastfeed, black and did not live with their partner, compared with the BFH group. However, these maternal variables did not influence BFFHL, as shown in Table 2. Variables associated with non-breastfed infants in the first hour of life in the adjusted model were born in a non-BFH hospital, by caesarean section and not having skin-to-skin contact (Table 2).
Table 2.
Proportion of non-breastfed infants in the first hour of life (nBFFHL), crude prevalence ratios (PR c ) and prevalence ratios adjusted (PR adj ) according to selected variables, São Paulo city, 2018
| Variables | PR c | PR adj | |||||
|---|---|---|---|---|---|---|---|
| n | nBFFHL (%) | P * | OR | 95 % CI | OR | 95 % CI | |
| Groups | |||||||
| BFH | 375 | 33·3 | 0·000 | 1 | 1 | ||
| Non-BFH | 267 | 49·4 | 1·48 | 1·16, 1·89 | 1·41 | 1·02, 1·96 | |
| Delivery routines | |||||||
| Type of delivery | |||||||
| Vaginal | 419 | 28·2 | 0·000 | 1 | 1 | ||
| Caesarean | 223 | 62·3 | 2·21 | 1·73, 2·83 | 2·05 | 1·48, 2·85 | |
| Skin-to-skin | |||||||
| Yes | 130 | 13·1 | 0·000 | 1 | 1 | ||
| No | 512 | 46·9 | 3·58 | 2·19, 5·86 | 3·93 | 1·90, 8·17 | |
| Maternal characteristics | |||||||
| Skin colour | |||||||
| Black | 134 | 42·5 | 0·780 | – | – | ||
| Brown | 249 | 39·0 | |||||
| White | 238 | 39·5 | |||||
| Living with partner | |||||||
| Yes | 500 | 38·8 | 0·235 | – | – | ||
| No | 135 | 44·4 | |||||
| Intention to breastfeed | |||||||
| Yes | 377 | 37·7 | 0·092 | 1 | – | ||
| No | 257 | 44·4 | 1·18 | 0·92, 1·51 | |||
BFH, Baby-Friendly Hospital; non-BFH, non-Baby-Friendly Hospital.
χ2 test comparing intervention and control groups.
Table 3 shows the number of late neonatal deaths avoided by BFFHL in both groups, estimated by the PF. The increase in BFFHL promoted by the BFHI resulted in a reduction of forty-nine all-cause deaths and fourteen more deaths from infections, equivalent to potential reduction of 13 and 13·1 %, respectively, in late neonatal mortality from all causes and from infections in São Paulo city during 2016 (Table 3).
Table 3.
Estimates of deaths averted in intervention group (Baby-Friendly Hospital, BFH) and comparison group (non-Baby-Friendly Hospital, non-BFH), according to mortality indicators, São Paulo city, 2018
| Indicators | Cases in São Paulo* (2016) | BFH | Non-BFH | BFH difference | Infant mortality reduction (%) | ||
|---|---|---|---|---|---|---|---|
| PF | Cases avoided | PF | Cases avoided | ||||
| Late neonatal mortality rate | 377 | 0·2948 | 158 | 0·2244 | 109 | 49 | 13·0 |
| Late neonatal mortality rate due to infections | 107 | 0·3015 | 46 | 0·2295 | 32 | 14 | 13·1 |
PF, preventive fraction.
Source: Department of Informatics of the Brazilian Unified Health System (http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/nrsp.def).
Direct costs of Baby-Friendly Hospital Initiative
The costs with human resources represented the highest proportion of the overall direct costs of births (68·8 %), followed by exams (11·6 %), medication (11·0 %) and other inputs (8·0 %). Direct costs related to BFHI training accounted only for 0·58 % of average cost of deliveries in hospitals participating in the initiative. Mean direct cost of births in the BFH group was significantly lower for both vaginal and caesarean delivery (Table 4).
Table 4.
Distribution of direct costs of deliveries in intervention group (Baby-Friendly Hospital, BFH) and comparison group (non-Baby-Friendly Hospital, non-BFH), according to cost categories (mean), São Paulo city, 2018
| Cost categories | Total sample ($US) | BFH ($US) | Non-BFH ($v) | P * |
|---|---|---|---|---|
| Overall cost | 259·44 | 252·78 | 268·78 | 0·001 |
| Vaginal delivery | 223·19 | 217·84 | 231·23 | 0·000 |
| VD2 | 200·71 | 200·40 | 201·36 | 0·421 |
| VD3 | 227·89 | 221·81 | 235·57 | 0·001 |
| VD5 | 280·05 | 335·99 | 265·53 | 0·000 |
| Caesarean section | 326·80 | 314·20 | 346·49 | 0·000 |
| CS2 | 276·79 | 272·01 | 285·71 | 0·015 |
| CS3 | 338·70 | 316·29 | 367·40 | 0·000 |
| CS5 | 371·98 | 372·15 | 371·64 | 0·893 |
BFH, Baby-Friendly Hospital; non-BFH, non-Baby-Friendly Hospital; VD2, vaginal delivery with up to 2 d of hospitalisation; VD3, vaginal delivery with 3–4 d of hospitalisation; VD5, vaginal delivery with 5 or more days of hospitalisation; CS2, caesarean section with up to 2 d of hospitalisation; CS3, caesarean section with 3–4 d of hospitalisation; CS5, caesarean section with ≥5 d of hospitalisation.
Mann–Whitney test for comparison of means between groups.
On average, vaginal delivery had approximately 45 % lower direct costs compared with caesarean section. Costs increased proportionally according to length of stay. BFFHL was associated with a shorter length of stay, resulting in a higher prevalence of vaginal delivery with up to 2 d of hospitalisation (RP = 1·89; 95 % CI 1·31, 2·70), in comparison with children who were not breastfed in the first hour of life. Consequently, the results pointed to a shorter length of stay in BFH for vaginal delivery (P = 0·010). Being born in BFH increased the prevalence of vaginal delivery with 2 d of hospitalisation (PR = 1·47; 95 % CI 1·05, 2·06). On the other hand, being born in non-BFH quadrupled the prevalence of vaginal delivery with ≥5 d of hospitalisation (RP = 3·96; 95 % CI 1·99, 7·87).
Baby-Friendly Hospital Initiative cost-effectiveness
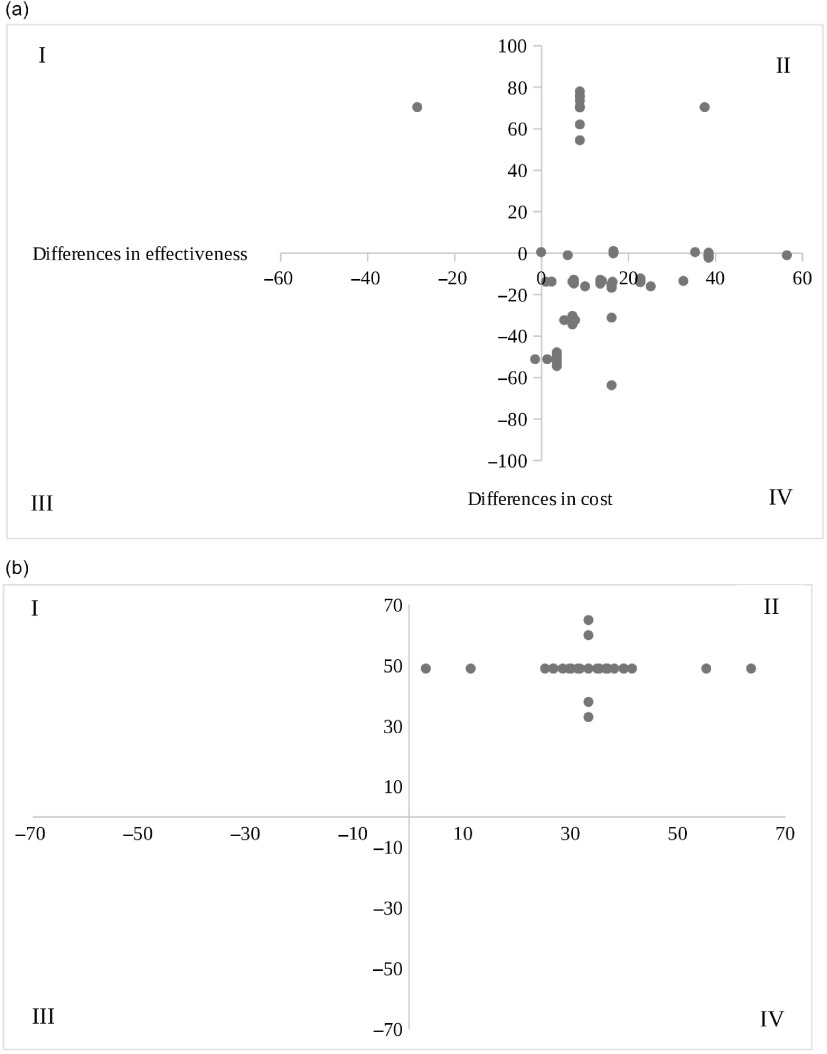
Figure 2 presents the cost-effectiveness diagrams for BFFHL and late neonatal deaths avoided, showing alternative scenarios resulting from changes in key parameters of costs and effectiveness that may result in deviations from the deterministic scenario.
Fig. 2.
Cost-effectiveness dispersion diagrams of breast-feeding during the first hour of life (a) and neonatal deaths avoided (b), São Paulo city, 2018.  , deterministic scenario;
, deterministic scenario;  , parameter variations; I: Quadrant I; II: Quadrant II; III: Quadrant III; IV: Quadrant IV
, parameter variations; I: Quadrant I; II: Quadrant II; III: Quadrant III; IV: Quadrant IV
Considering the intermediate outcome (BFFHL), the BFHI presented 76·2 % of the alternative cases resulting from parameter variations in the quadrant IV (dominance), which means that changes in initial conditions may result in lower cost and greater effectiveness for 76·2 % of the situations (Fig. 2(a)).
Referring to the final outcome (late neonatal deaths avoided), all simulated scenarios due to variations in parameters were included in quadrant II, which means that changes in costs and/or outcomes may generate slightly higher costs associated with greater effectiveness in relation to the deterministic scenario (Fig. 2(b)).
Incremental cost-effectiveness ratio
ICER in the intermediate outcome was negative, indicating that BFH was dominant compared with non-BFH promoting a 16·1-percentage point increase in BFFHL prevalence (+31·8 %) at a lower cost (Table 5). Regarding avoided infant deaths, positive ICER indicated a slightly higher cost in the BFH group, with a $US 0·68 increase in costs for prevented deaths (Table 5).
Table 5.
Cost-effectiveness ratio (CER) and incremental cost-effectiveness ratio (ICER) of breast-feeding in the first hour of life (BFFHL) and neonatal mortality, according to cost categories, São Paulo city, 2018
| BFFHL | ||||||
|---|---|---|---|---|---|---|
| Groups | Cost ($US) | Incremental cost ($US) | Effectiveness BFFHL (%) | Incremental effectiveness | CER ($US/pp)* | ICER |
| BFH | 252·79 | −15·99 | 66·7 | 16·1 | 3·79 | −0·99 (dominant) |
| Non-BFH | 268·78 | 50·6 | 5·31 | |||
| VDBFH | 217·84 | −13·39 | 81·6 | 22·7 | 2·67 | −0·59 (dominant) |
| VDnBFH | 231·23 | 58·9 | 3·93 | |||
| CSBFH | 314·20 | −32·29 | 40·4 | 7·1 | 7·78 | −4·55 (dominant) |
| CSnBFH | 346·49 | 33·3 | 10·41 | |||
BFH, Baby-Friendly Hospital; non-BFH, non-Baby-Friendly Hospital; VDBFH, vaginal delivery in the BFH group; VDnBFH, vaginal delivery in the non-BFH group; CSBFH, caesarean section in the BFH group; CSnBFH, caesarean section in the non-BFH group.
$US/pp = cost per percentage points.
$US/ac = cost per avoided cases.
The ICER in the final outcome was positive because the final cost obtained by summing the decision tree parameters probabilities was influenced by the higher probability of being born in BFH (BFH 0·58 and non-BFH 0·42) and higher probability of caesarean delivery in the BFH group (BFH 0·36 and non-BFH 0·33), according to decision tree in Appendix 2 in the online supplementary material.
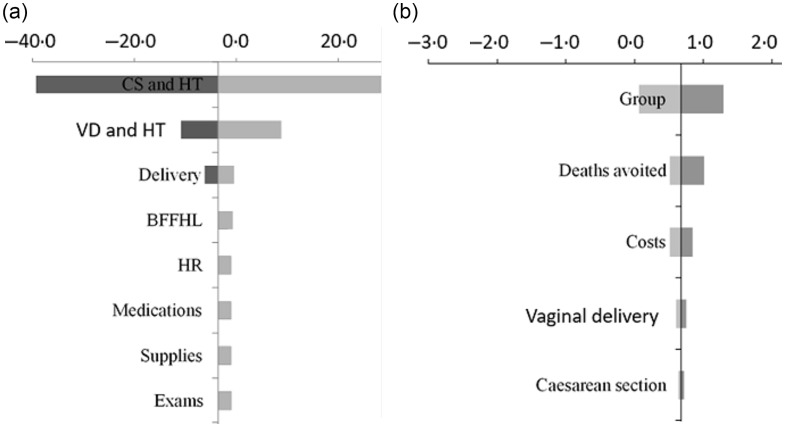
The sensitivity analysis represented the changes in ICER due to variations in costs and end points, according to categories for promotion of BFFHL and reduction in late neonatal mortality (Fig. 3).
Fig. 3.
Tornado diagrams with variations of incremental cost-effectiveness ratio due to cost parameters of breast-feeding in the first hour of life (BFFHL) and avoided cases of late neonatal mortality from all causes, São Paulo city, 2018. CS and HT, variation in length of stay for caesarean section deliveries; VD and HT, variation in length of stay for vaginal deliveries; delivery, variation in the prevalence of type of delivery; HR, variation of costs due to human resources; group, cost-effectiveness variation as a function of birth probability in the Baby-Friendly Hospitals (BFH) and non-BFH groups
The length of hospitalisation, especially combined with caesarean section delivery, was the parameter with the highest influence in ICER for BFFHL (Fig. 3(a)), in comparison with changes in other parameters determining ICER. The results of univariate sensitivity analysis for reduction in neonatal mortality (Fig. 3(b)) indicated that variations in the probability of being born in the BFH or non-BFH group would be the parameter with the greatest influence on the ICER, followed by variations in effectiveness. However, considering the scenarios projected, variations in ICER reached the maximum amount of $US 1·30 per death avoided, showing that cost-effectiveness ratios estimated in the intervention were robust to changes in costs and outcomes.
Discussion
The study showed evidence of the effectiveness of the BFHI in increasing the prevalence of BFFHL by approximately 32 %. This result corroborates global evidence that the BFHI is one of the most effective interventions to improve the prevalence of BFFHL(6,8,26). Other studies in Brazil have also supported this effect, such as the study by Carvalho et al.(27), which reported that the BFHI doubled the prevalence of BFFHL and the study by Venancio et al.(15), which analysed a nationally representative sample and found that the BFHI increased the BFFHL by 9 %; the impact, although minor, is significant. The data reinforce the importance of this strategy, considering the potential of the BFFHL in reducing neonatal mortality(23,28,29).
The reduction of neonatal mortality rate as the result of BFFHL promotion by the BFHI is consistent with evidence from other studies that early initiation of breast-feeding is among the most effective interventions to reduce neonatal mortality(23,28–31). Therefore, it is important to foster investments in public policies that promote adherence to early breast-feeding initiation, such as the BFHI. Corroborating the findings, Silva et al.(12), analysing data from the National Survey on Breastfeeding in Brazil, found that BFHI was effective in increasing the BFFHL by 11·7 % and potentially contributing to reduction of 4·2 % of late neonatal deaths in Brazil. The increase in BFFHL was higher in the current study, with greater effect in mortality rate reduction. This evidence allows to infer that higher effectiveness of the BFHI in raising the prevalence of BFFHL represents larger proportion of children’s lives saved. The late neonatal mortality rate in Brazil was reduced from 5·4 in 1990 to 2·2 per 1000 live births in 2015(32). There were 167 276 live births registered in São Paulo municipality during 2016, and late neonatal mortality rate was 2·25 per thousand(24). Considering that the neonatal component corresponds to 70 % of infant mortality in Brazil, infections being the second leading cause of death(32), especially in late neonatal period(28), the potential of BFHI in reducing neonatal mortality confirms its relevance as public policy to promote child survival.
Direct cost of deliveries in the BFH group was significantly lower compared with non-BFHI. Since the breast-feeding in the first hour was associated with a shorter length of stay and lower costs, this difference may be partly attributed to the increase in the BFHHL promoted by the BFHI. Thus, considering the effectiveness of BFHI for promotion of breast-feeding in the first hour, the wide adoption of the initiative in Brazilian hospitals may provide benefits to the population and also to the national health system.
Furthermore, the direct costs involved in the development and promotion of BFHI through the assignment of one health professional responsible for monitoring the initiative and annual training of health professionals’ teams were included in the estimation of costs within the study, comprising approximately 0·6 % of direct costs per birth in a BFH. Nevertheless, the births in a BFH remained lower than in a non-BFH, which did not include these additional costs. This suggests that the direct costs of maintaining BFHI would not be a barrier to promoting breast-feeding at the hospital level.
A study conducted by Oliveira(33) in Rio de Janeiro investigated the costs of delivery within a BFH in Brazil and, when assessing the direct costs of childbirth in a birth centre, the authors projected that if all deliveries occurred in a BFHI facility, there would be an annual saving of approximately $ 630 000 for childbirth. There is lack of cost estimates referring to BFHI in the literature worldwide. A systematic review recently published(10) on costs for implementation and enhancement of interventions for breast-feeding identified only five studies focusing on BFHI. Only two studies, both performed in the USA, compared the costs of births in BFH and non-BFH(34,35).
Dellifraine et al.(34) noted that the BFHI had a slightly higher cost (1·6–5·0 %) than other hospitals; however, the difference was not statistically significant. When analysing hospital data from twenty US states, Allen et al.(35) found an increase in costs according to the number of BFHI steps completed (also not statistically significant).
The cost-effectiveness analysis of the BFHI for promotion of BFFHL indicated a negative ICER, showing that the BFHI was dominant in relation to non-BFH, that is, highly cost-effective with important results for mothers who went through caesarean delivery, even considering additional costs for annual health professionals training, according to initiative’s recommendations. The shorter length of stay (2 d) and absence of complications were situations with higher cost-effectiveness ratios (in favour of BFHI), while the prolonged length of stay of the mother and/or child increased the costs and reduced cost-effectiveness. Similarly, results from a systematic review performed by Renfrey et al.(36) showed evidence that trained health professionals’ support in hospitals was potentially cost-saving for promotion of breast-feeding, increasing the quality-adjusted life years.
Contrary to BFFHL, the cost-effectiveness ratio for neonatal mortality had a positive ICER through estimation of costs in comparison with outcomes probabilities within decision tree modelling. The costs were influenced by a higher probability of being born within BFH in combination with a higher probability of caesarean delivery in the BFH, according to information obtained in the cohort. Compared with similar costs prevailing in other branches of the decision tree, the cost-effectiveness ratio resulted in slightly higher costs and positive ICER within the BFH group. Therefore, scenarios involving equal probability of birth in BFH and non-BFH with equal probability of caesarean section in both groups showed a negative ICER.
It is important to highlight that the effectiveness was also higher within the BFH group, including potential scenarios in sensitivity analysis located in quadrant II, which involves decision-making based on cost-effectiveness ratios thresholds. Considering the absence of threshold for health interventions established in Brazil and the low ICER obtained ($US 0·68 per neonatal death avoided), the BFHI provides extensive social benefits in costs in relation to other health programmes.
Additionally, direct costs established in the current study considered only the payers’ perspective (Brazilian government), that is, the inclusion of potential future hospitalisation costs due to health problems associated with late neonatal mortality in the absence of early breast-feeding could impose supplementary costs in the non-BFH group, resulting in further economic advantages to the initiative. Evidence in recent studies shows that breast-feeding potentially prevents 623 000 premature deaths of children and 20 000 premature deaths of women worldwide(1), highlighting the burden imposed by the absence of breast-feeding and early weaning in health systems and societies(1,2,25).
There was lack of evidence regarding cost-effectiveness studies related to the BFHI in the literature review performed, and the existing information on cost-effectiveness was related only to general breast-feeding promotion activities in three countries (Brazil, Honduras and Mexico)(37). Horton et al.(37) estimated morbidity and mortality cases avoided due to diarrhoea and respiratory infections and disability-adjusted life years, concluding that the cost-effectiveness ratio of the programmes evaluated was comparable with gains in death cases avoided due to immunisations, vitamin A supplementation and short-term treatment of tuberculosis(38).
The main limitation of the study refers to sample representativeness, since it encompasses only population using public hospitals. On the other hand, most BFHI in Brazil are public hospitals, that is, the methodological criteria used for sample selection encompassed major part of the target group. It is important to highlight the originality of the study in conducting a prospective cohort to perform a cost-effectiveness analysis of BFHI in promoting early breast-feeding and reducing neonatal mortality. Given the unavailability of information to compare the cost-effectiveness analysis, future investigations should focus on collecting long-term longitudinal data on costs and impacts on health outcomes referring to BFHI implementation. This should be studied especially considering indirect and societal costs due to early weaning, and benefits attributable to breast-feeding in the prevention of diseases and reduction of future health care costs.
Conclusion
The study provides the first cost-effectiveness analysis of the BFHI, conducted in Brazil in prospective cohort of mothers to present data on cost-effectiveness of breast-feeding outcomes, particularly in the promotion of adherence in the first hour of life and in the reduction of late neonatal mortality rates. The results showed dominance of the initiative in deterministic and sensitivity analysis scenarios for early promotion of breast-feeding and also its cost-effective results in the reduction of late neonatal mortality rates. The evidence represents an advance in economic assessment of public health programmes that require low level of expenditures for investments in its expansion and sustainability, resulting in health, social and economic gains in short, medium and long term. Based on findings of the current study, considering estimate of deaths avoided by BFHI between 7 and 28 d after birth (forty-nine deaths averted), late neonatal mortality rate could be reduced to approximately 1·96 per thousand in the municipality during 2016 due to adoption of BFHI in all hospitals, representing a reduction of 13 % in this infant mortality rate component.
Acknowledgements
Acknowledgements: The authors would like to thank São Paulo Research Foundation. Financial support: The current work was supported by São Paulo Research Foundation, process no. 2016/11688-1. São Paulo Research Foundation had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: O.L.O.S. contributed to conception and design of the study, carrying it out data acquisition, analysis and interpretation of the data and writing the article. M.F.R. contributed to conception and design of the study, analysis and interpretation of the data, writing the article and final approval. F.M.S. contributed to analysis and interpretation of the data, writing the article and final approval. G.B. contributed to analysis and interpretation of the data and writing the article. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Research Ethics Committee of the School of Public Health at University of São Paulo under no. 1.811.327/2016. Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020001871.
click here to view supplementary material
References
- 1. Victora CG, Bahl R, Barros AJD et al. (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490. [DOI] [PubMed] [Google Scholar]
- 2. Rollins NC, Bhandari N, Hajeebhoy N et al. (2016) Why invest, and what it will take to improve breastfeeding practices? Lancet 387, 491–504. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) (2013) Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. Geneva: WHO. [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) (2017) National Implementation of the Baby-Friendly Hospital Initiative. Geneva: World Health Organization. https://www.who.int/nutrition/publications/infantfeeding/bfhi-national-implementation2017/en/ (accessed October 2019). [Google Scholar]
- 5. World Health Organization (WHO) (2017) Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/handle/10665/259386/9789241550086-eng.pdf;jsessionid=109CB9FDBDDA228B01678DD25317BB55?sequence=1 (accessed September 2018). [PubMed] [Google Scholar]
- 6. Sinha B, Chowdhury R, Sankar MJ et al. (2015) Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Paediatr 104, 114–135. [DOI] [PubMed] [Google Scholar]
- 7. Pan American Health Organization (PAHO) (2016) Baby-Friendly Hospital Initiative in Latin America and the Caribbean: Current Status, Challenges and Opportunities. Washington, DC: PAHO. [Google Scholar]
- 8. Pérez-Escamilla RP, Martinez JL & Segura-Pérez S (2016) Impact of the baby-friendly hospital initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr 12, 402–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO) (2018) Implementation Guidance: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: The Revised Baby-Friendly Hospital Initiative. Geneva: World Health Organization. [Google Scholar]
- 10. Carroll G, Safon C, Buccini G et al. (2020) A systematic review of costing studies for implementing and scaling-up breastfeeding interventions: what do we know and what are the gaps? Health Policy Plan 35, 461–501. [DOI] [PubMed] [Google Scholar]
- 11. United Nations International Children’s Emergency Fund/Organisation Mondiale de la Santé (2017) Country experiences with the Baby-friendly Hospital Initiative. Compendium of case studies from around the world. https://www.unicef.org/nutrition/files/BFHI_Case_Studies_FINAL.pdf (accessed October 2018).
- 12. Silva OLO, Rea MF, Venancio SI et al. (2018) The baby-friendly hospital initiative: increasing breastfeeding and decreasing infant mortality in Brazil. Rev Bras Saude Mater Infant 18, 481–489. [Google Scholar]
- 13. World Health Organization (WHO) (2010) Health Systems Financing: The Path to Universal Coverage. Geneva: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brazilian Ministry of Health (BR) (2014) Methodological Guidelines: Economic Evaluation Guideline, 2nd ed. Brasília: Brazilian Ministry of Health. [Google Scholar]
- 15. Venancio SI, Saldiva SRDM, Escuder MML et al. (2011) The baby-friendly hospital initiative shows positive effects on breastfeeding indicators in Brazil. J Epidemiol Community Health 66, 914–918. [DOI] [PubMed] [Google Scholar]
- 16. Brazilian Ministry of Health (BR) (2009) II Breastfeeding Prevalence Survey in Brazilian Capitals and in the Federal District. Brasília, DF. http://bvsms.saude.gov.br/bvs/publicacoes/pesquisa_prevalencia_aleitamento_materno.pdf (accessed June 2019).
- 17. Sarti FM & Cyrillo DC (2010) Cost assessment in health economics projects. In Health Technology Assessment [ME Nita, ACC Campino, SR Secoli et al., editors]. Porto Alegre: Artmed, 600p. [Google Scholar]
- 18. Gargiullo PM, Wilson HG & Rothenberg RB (1995) Confidence intervals, hypothesis tests, and sample sizes for the prevented fraction in cross-sectional studies. Stat Med 14, 5172. [DOI] [PubMed] [Google Scholar]
- 19. Walter SD, Hsieh CC & Liu Q (2007) Effect of exposure misclassification on the mean squared error of population attributable risk and prevented fraction estimates. Stat Med 26, 4833–4842. [DOI] [PubMed] [Google Scholar]
- 20. Miettinen OS (1974) Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 99, 325–332. [DOI] [PubMed] [Google Scholar]
- 21. Wilson LF, Green AC & Kendall BJ (2015) Cancers prevented in Australia in 2010 through the consumption of aspirin. Aust N Z J Public Health 39, 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whiteman DC, Webb PM & Green AC (2015) Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 39, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Debes AK, Kohli A, Walker N et al. (2013) Time to initiation of breastfeeding and neonatal mortality and morbidity: a systematic review. BMC Public Health 13, Suppl. 3, S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prefeitura de São Paulo – TabNet (2018) TABNET. http://tabnet.saude.prefeitura.sp.gov.br/cgi/tabcgi.exe?secretarias/saude/TABNET/minf/mortinf.def (accessed October 2019).
- 25. Bartick M & Reinhold A (2010) The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 125, 1048–1056. [DOI] [PubMed] [Google Scholar]
- 26. Kramer MS, Chalmers B, Hodnett ED et al. (2001) Promotion of breastfeeding intervention trial (PROBIT): a randomized trial in the republic of Belarus. JAMA 285, 413–420. [DOI] [PubMed] [Google Scholar]
- 27. Carvalho ML, Boccolini CS, Oliveira MIC et al. (2016) The baby-friendly hospital initiative and breastfeeding at birth in Brazil: a cross sectional study. Reproduct Health 13, 207–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan J, Vesel L, Bahl R et al. (2015) Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity: a systematic review and meta-analysis. Matern Child Health J 19, 468–479 [DOI] [PubMed] [Google Scholar]
- 29. Boccolini CS, Carvalho ML, Oliveira MIC et al. (2013) Breastfeeding during the hour of life and neonatal mortality. J Pediatr 89, 131–136. [DOI] [PubMed] [Google Scholar]
- 30. Smith ER, Hurt L, Chowdhury R et al. (2017) Delayed breastfeeding initiation and infant survival: a systematic review and metaanalysis. PLOS ONE 12, e018072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phukan D, Ranjan M & Dwivedi LK (2018) Impact of timing of breastfeeding initiation on neonatal mortality in India. Int Breastfeed J 3, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leal MC, Szwarcwald CL, Almeida PVB et al. (2018) Reproductive, maternal, neonatal and child health in the 30 years since the creation of the Brazilian Unified Health System (SUS). Ciência Saúde Coletiva 23, 1915–1928. [DOI] [PubMed] [Google Scholar]
- 33. Oliveira FA (2014) Direct costs of delivery with related obstetrical nursing practice in Birth Center. Esc Anna Nery 18, 421–427. [Google Scholar]
- 34. DelliFraine J, Langabeer J II, Williams JF et al. (2011) Cost comparison of baby friendly and non-baby friendly hospitals in the United States. Pediatrics 127, 989–994. [DOI] [PubMed] [Google Scholar]
- 35. Allen JA, Longenecker HB, Perrine CG et al. (2013) Baby-friendly hospital practices and birth costs. Birth 40, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Renfrey MJ, Craig D, Dyson L et al. (2009) Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis. Health Technol Assess 13, 1–146. [DOI] [PubMed] [Google Scholar]
- 37. Horton S, Sanghvi T, Phillips M et al. (1996) Breastfeeding promotion and priority setting in health. Health Policy Plan 1, 156–168. [DOI] [PubMed] [Google Scholar]
- 38. Pérez-Escamilla R (2007) Evidence based breast-feeding promotion: the baby-friendly hospital initiative. J Nutr 137, 484–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020001871.
click here to view supplementary material