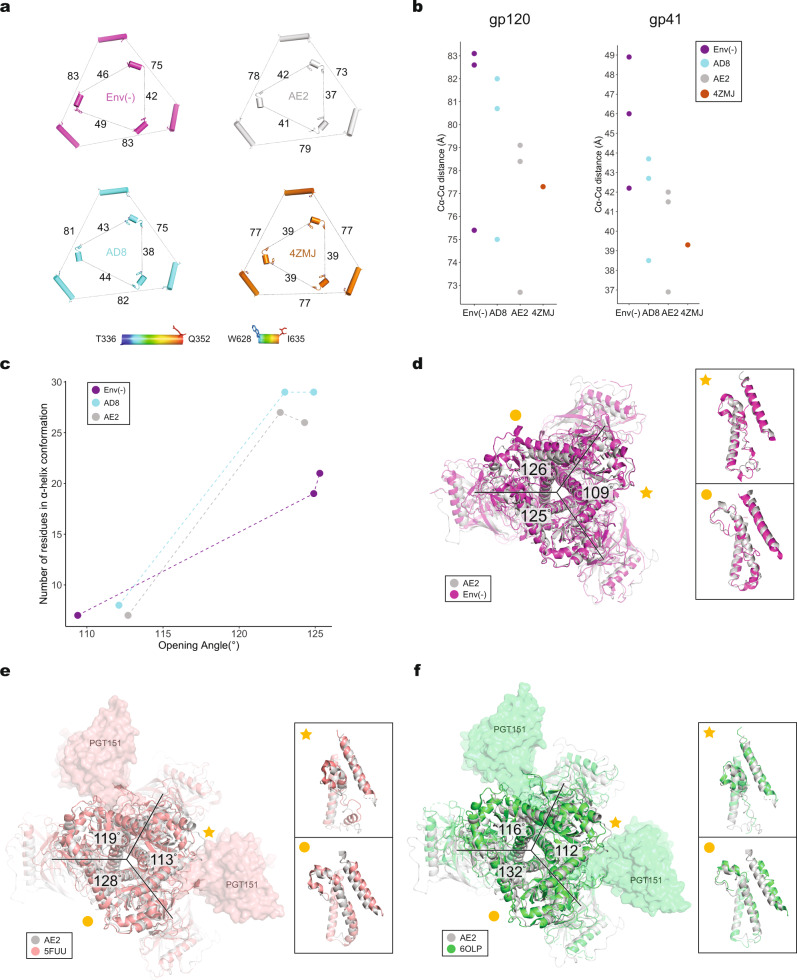
Fig. 3. Comparison of trimer geometry among different Env structures.
a The interprotomer distances (in Å) between selected Cα atoms (Thr 336 and Gln 352) of gp120 (outer triangles) and Cα atoms (Trp 628 and Ile 635) of gp41 (inner triangles) are shown for the AD8 and AE2.1 Envs, Env(–) (PDB 7N6U) and an unliganded SOSIP Env trimer (PDB 4ZMJ). b The Cα–Cα distances shown in (a) are plotted for the gp120 and gp41 subunits. c The relationship between helicity of the FPPR–HR1N region and the opening angle of the AD8, AE2.1 and Env(–) trimers is shown. The x axis represents the opening angle for each of the interprotomer interfaces, measured in PyMOL. The y axis represents the number of residues in an α-helical conformation for the FPPR–HR1N region (residues Ser 534–Val 570) associated with an interprotomer interface. d The AE2.1 and Env(–) trimer models were superposed, based on alignment of AE2.1 gp120 (Chain E) with Env(–) gp120 (Chain B). The AE2.1 Env is colored gray and the Env(–) trimer is colored magenta. The opening angles of the Env(–) interprotomer interfaces are shown. e Comparison of the AE2.1 Env and the PGT151-bound HIV-1JR-FL EnvΔCT structures (PDB 5FUU). The AE2.1 Env Chain E structure (in gray) is superposed on Chain C of the PGT151-bound HIV-1JR-FL EnvΔCT structure (in salmon), with the PGT151 Fabs shown. The opening angles between the protomers of the PGT151-bound HIV-1JR-FL EnvΔCT trimer are shown. f Comparison of the AE2.1 Env and the PGT151-bound HIV-1AMC011 EnvΔCT structures (PDB 6OLP). The AE2.1 Env Chain E structure (in gray) is superposed on Chain C of the PGT151-bound HIV-1AMC011 EnvΔCT structure (in green), with the PGT151 Fabs shown. The opening angles between the protomers of the PGT151-bound HIV-1AMC011 EnvΔCT trimer are shown. d–f close-up side views of the interprotomer interfaces with a smaller opening angle (star) and a larger opening angle (circle) are shown in the insets. The close-up views show the gp41 fusion peptide, FPPR, HR1N and HR1C regions from the superposed protomers and the α9 helix from the adjacent protomer.