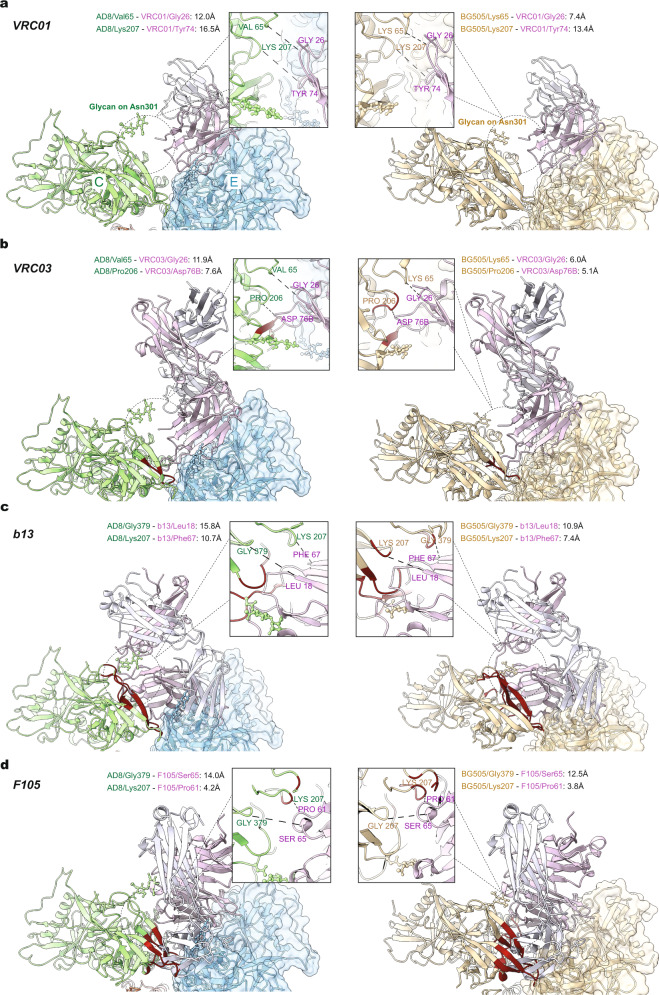
Fig. 6. Antibody docking on a symmetric Env trimer and the more open interface of an asymmetric Env trimer.
Models of antibody-bound complete Env trimers or subunits were aligned with the asymmetric AD8 trimer model or the symmetric HIV-1BG505 SOSIP trimer model (PDB 4ZMJ), according to the gp120 chain. The gp120 of the Env–antibody complex was aligned to Chain E gp120 of the AD8 Env trimer that, along with Chain C gp120, flank an interprotomer interface with a larger opening angle (124°). AD8 models (Chain E is blue and Chain C is green) are on the left; HIV-1BG505 SOSIP (wheat) models are on the right. The antibody heavy and light chains are colored with different shades of purple to allow them to be distinguished. The Env protomer bound by the antibody is shown with a molecular surface, and conflict residues in the adjacent Env protomer are shown in red. Conflict residues are defined as containing backbone atoms within 4 Å of any antibody atom. Details of the region of closest approach of antibody and the adjacent Env protomer are shown in the insets. The distances shown are measured between Cα atoms of selected residues in ChimeraX61. The CD4BS antibodies include: a VRC01 (PDB 5FYK); b VRC03 (PDB 3SE8); c b13 (PDB 3IDY); d F105 (PDB 3HI1). VRC01 and VRC03 are CD4BS bNAbs, whereas b13 and F105 are CD4BS pNAbs.