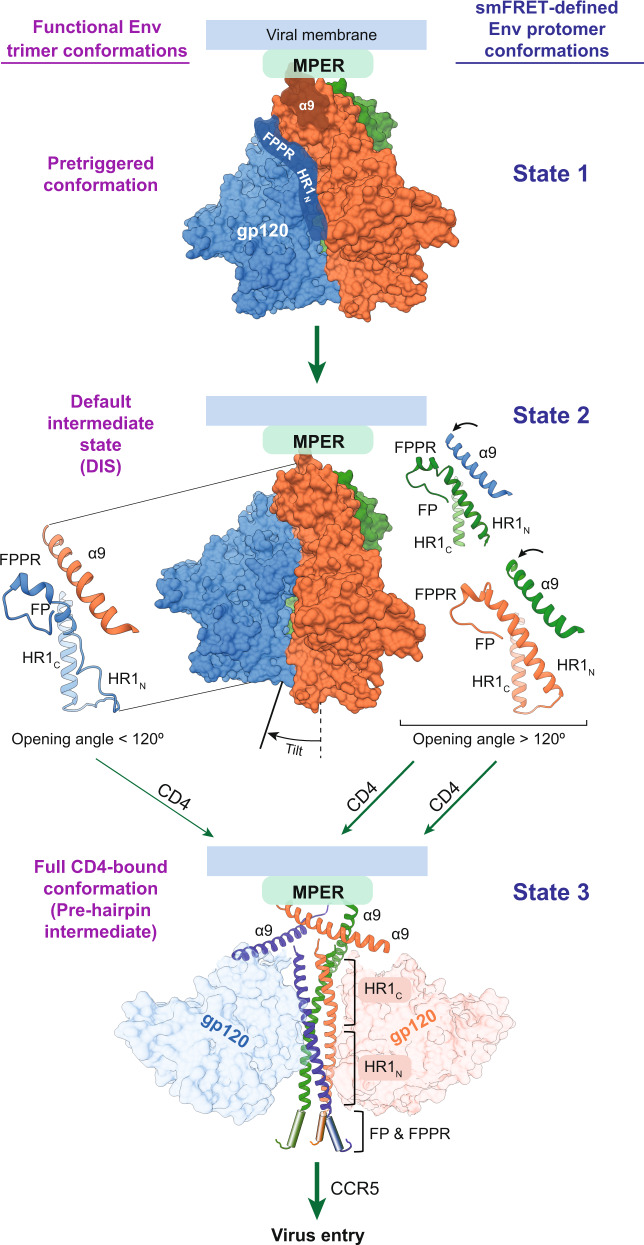
Fig. 7. A model involving an asymmetric HIV-1 Env trimer in virus entry.
A model of the early steps in HIV-1 entry is shown, with the Env protomers colored as in Fig. 1 (MPER, gp41 membrane-proximal external region; α9, gp41 region spanning residues 628-664; FP, gp41 fusion peptide; FPPR, gp41 fusion peptide-proximal region; HR1N and HR1C, gp41 heptad repeat N- and C-terminal regions, respectively). The names of the functional Env trimer conformations are shown to the left of the figures, and the smFRET-defined conformations of the Env protomers are shown to the right of the figures5–7. In the pretriggered Env conformation, the close association of the gp41 α9 region with the MPER hypothetically modulates the interaction between α9 and FPPR on the neighboring protomer. In turn, the interaction of FPPR and the adjacent HR1N region with the gp120 inner domain contributes to the maintenance of the pretriggered conformation. Spontaneous transitions between the pretriggered conformation and the asymmetric default intermediate state (DIS) are governed by HIV-1 strain-dependent variables6,10,11,34. Tilting of the DIS Env in the membrane is allosterically coupled to asymmetric displacement of the α9 helices, an increase in two of the opening angles between the protomers, and transitions of the associated HR1N regions into helical conformations. In the asymmetric DIS, two protomers can bind CD4 with less steric hindrance and have more helical HR1N regions. Thus, the DIS is predisposed to rearrange into the full CD4-bound conformation (the prehairpin intermediate), where the newly formed HR1N helices extend the HR1C coiled coil, relocating the fusion peptide (FP) closer to the target cell membrane15. Binding the second receptor, CCR5, permits the prehairpin intermediate to form the six-helix gp41 bundle that mediates membrane fusion and virus entry. The six-helix bundle is composed of the HR1 coiled coil (HR1N + HR1C) and the HR2 helices, which include the α9 helix16–18.