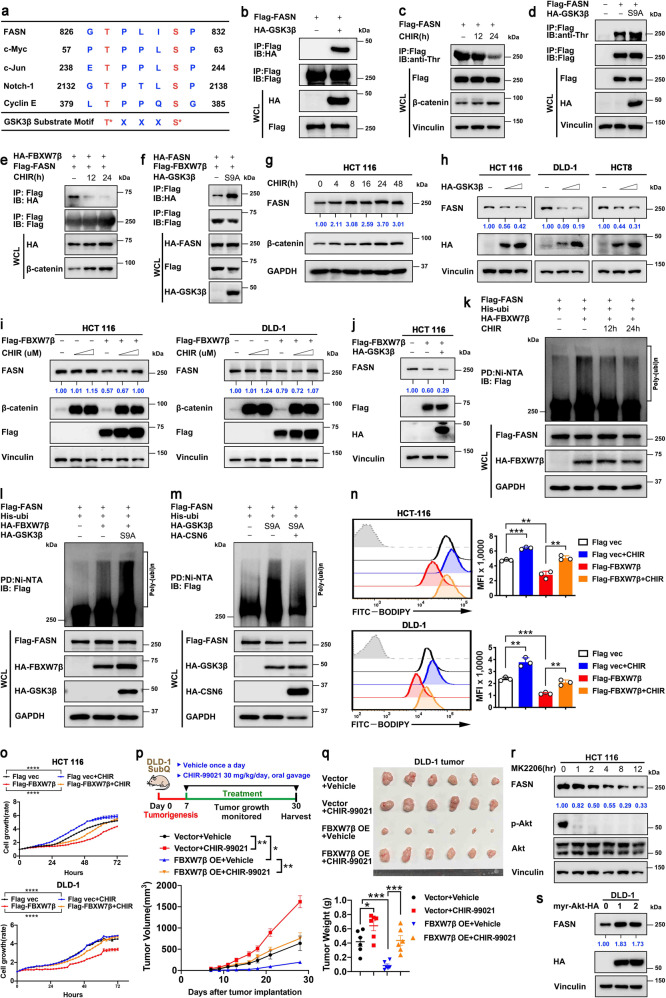
Fig. 4.
GSK3β-mediated FASN phosphorylation enhances FBXW7β-mediated ubiquitination/degradation of FASN and inhibits lipogenesis. a Sequence alignment of GSK3β substrate consensus sequences (S/TXXXS/T) and FBXW7β binding motifs of FASN. The reported GSK3β substrates (c-Myc, c-Jun, Notch-1, Cyclin E) are shown. Red, phospho-acceptor residue; blue, basic residue. X denotes any kind of residue. b HEK293T cells expressing Flag-FASN were co-transfected with HA-GSK3β. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and immunoblotted with the indicated antibodies. c HEK293T cells expressing Flag-FASN were treated with GSK3β inhibitor CHIR (4 μM) for the indicated time. DMSO was used as a vehicle control. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and immunoblotted with anti-phosphorylated-threonine (p-Thr) antibody. CHIR treatment efficiency was tested by β-catenin. d HEK293T cells were transfected with Flag-FASN together with or without HA-tagged constitutively active mutant (S9A) of GSK3β. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and immunoblotted with anti-phosphorylated-threonine (p-Thr) antibody. e HEK293T cells were co-transfected with Flag-FASN and HA-FBXW7β. Where indicated, CHIR (4 μM) was added for 12 h or 24 h before harvesting. DMSO was used as a vehicle control. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and immunoblotted with the indicated antibodies. f HEK293T cells were co-transfected with HA-FASN and Flag-FBXW7β in the presence or absence of HA-GSK3β (S9A). Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and immunoblotted with the indicated antibodies. g HCT 116 cells were treated with CHIR (4 μM) for the indicated time. DMSO was used as a vehicle control. Cell lysates were immunoblotted with the indicated antibodies. h Indicated cells were transfected with increased dose of HA-tagged GSK3β. Cell lysates were immunoblotted with the indicated antibodies. i HCT 116 or DLD-1 cells were transfected with or without Flag-FBXW7β. Increasing dose of CHIR (0/4/8 μM) was added for 24 h before harvest. DMSO was used as a vehicle control. Cell lysates were immunoblotted with the indicated antibodies. j HCT 116 were transfected with the indicated plasmids. Cell lysates were immunoblotted with the indicated antibodies. k HEK293T cells were transfected with the indicated plasmids and treated with CHIR (4 μM) for the indicated time. MG132 (20 μM) was added to the cells 6 h before they were harvested. The ubiquitinated FASN proteins were pulled down with nickel beads (Ni-NTA) and immunoblotted with an anti-Flag antibody. l, m HEK293T cells were transfected with the indicated plasmids. MG132 (20 μM) was added to the cells 6 h before they were harvested. The ubiquitinated FASN proteins were pulled down with nickel beads (Ni-NTA) and immunoblotted with an anti-Flag antibody. n HCT 116 cells transfected with indicated plasmids were treated with or without CHIR (4 μM) for 24 h and then stained with BODIPY 493/503 for quantification of intracellular lipids. Representative histograms and mean fluorescence intensities (MFI) were shown. o Cell growth assay generated from HCT 116 cells transfected with indicated plasmids and then treated with or without CHIR (4 μM). p Schematic design for DLD-1 subcutaneous tumor model (top). Tumor growth curves of indicated DLD-1 tumors (n = 6 mice per group) were shown (bottom). q The resulting tumors generated in p were resected and photographed at the end of the experiment (top). Tumor weights of indicated DLD-1 tumors were shown (bottom). r HCT 116 cells were treated with AKT inhibitor MK2206 (2 μM) for the indicated time. Cell lysates were immunoblotted with the indicated antibodies. s DLD-1 cells were transfected with increased dose of constitutively active form of Akt (myr-Akt). Cell lysates were immunoblotted with the indicated antibodies. All values are expressed as means ± SD. ns not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; as determined by two-way ANOVA (o, p) or by one-way ANOVA (n, q)