Table 2.
Four licensed JAKis for RA treatment
| JAKis | Main trials | Chemical structure | T1/2 | Excretion | Clinically used doses | ref. |
|---|---|---|---|---|---|---|
| Baricitinib | Phase III, Genovese et al. (RA-BEACON); Phase III, Dougados et al. (RA-BUILD); Phase III, Fleischmann et al. (RA-BEGIN); and Phase III, Taylor et al. (RA-BEAM) | 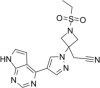 |
9–13 h | Urine75%, and Stool20% | 2 mg/4 mg | 475–478 |
| Tofacitinib | ORAL program: Phase III, Fleischmann et al. (ORAL solo); Phase III, Burmester et al. (ORAL step); Phase III, Vollenhoven et al. (ORAL-standard); Phase III, Kremer et al. (ORAL sync); Phase III, van der Heijde et al. (ORAL scan); and Phase III, Lee et al. (ORAL start) | 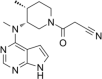 |
3 h | Urine51%, and Stool13% | 5 mg/10 mg | 200–205 |
| Filgotinib | Phase III, Genovese et al,(FINCH 2 trial), and Extension Study of Phase II, Kavanaugh et al. (DARWIN 3) | 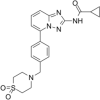 |
6 h | Urine87%, and Stool15% | 100 mg/200 mg | 177,178,196,197 |
| Upadacitinib | Phase III, Cohenet et al. (SELECT program) | 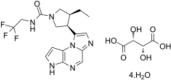 |
8–14 h | Urine24%, and Stool38% | 15 mg/30 mg | 261,262,265,266 |
