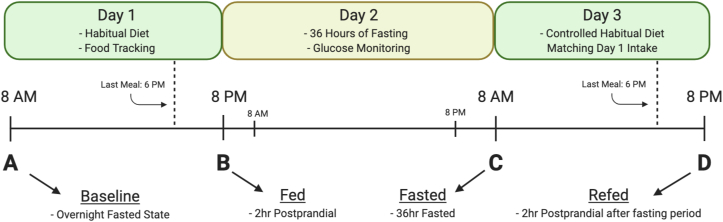
Figure 1.
Timeline of 3-d human fasting trial: 20 participants underwent a 3-d clinical trial consisting of 4 study visits in 4 distinct nutritional states to allow for the sensitive assessment of the effects of 36 h of fasting versus an overnight fasted state and the carryover effects of fasting onto the next eating day. On day 1, participants provided an overnight fasting baseline blood sample (A) and then went about their normal routine and habitual diet while tracking their food intake until 18:00 where participants ate their last meal. A 2-h postprandial fed blood sample was taken at 20:00 on day 1 (B), after which participants began their 36-h fast while being monitored for compliance via glucose monitors. At 08:00 on day 3, participants provided a 36-h fasted blood draw (C) and then were given a copy of their diet record from day 1 and instructed to eat the identical diet as they recorded on day 1. At 18:00 on day 3, participants ate their last meal and received a final 2-h postprandial re-fed blood draw (D) concluding the study.